Brian Chee1
1Northern Health Clinical School, The University of Melbourne, Melbourne, Australia
b.chee@student.unimelb.edu.au
Journal MTM 1:4:38-41, 2012
DOI:10.7309/jmtm.81
Introduction
This article begins with a case report illustrating the use of a generic mobile device in operating a proprietary medical device – the vagal nerve stimulation (VNS) therapy, used in the treatment of refractory epilepsy.
Case
Mr AP is a 20-year old man with an intellectual disability who suffers from intractable epilepsy since 8 years of age. He experiences brief atonic events very frequently. In addition to this, his seizure pattern consists of more prolonged generalised tonic-clonic seizures 2 – 3 times per week, and awake tonic events 2 – 4 times per week.
Despite trialling multiple anti-epileptic medications, his seizures remain poorly controlled and was progressively worsening over the past few years. His latest regimen was a combination of sodium valproate 550 mg, clonazepam 0.5 mg twice daily, and phenytoin 260 mg. An MRI scan showed generalised cerebral atrophy and peri-ventricular heteropia without any surgically amenable lesions. Hence, his treating neurologist agreed to a trial of vagal nerve stimulation therapy. The procedure was carried out by the Austin Hospital neurosurgical team under general anaesthesia.
Vagal Nerve Stimulation
Vagal nerve stimulation (VNS) is recognised as an adjunctive therapy for treatment-resistant epilepsy, used in more than 60,000 patients worldwide 1. The instrument is currently produced solely by Cyberonics, Inc. and consists of a pulse generator, which is placed in a surgically-created subcutaneous pocket in the left upper chest or anterior axillary fold. The generator delivers stimulation to the afferent fibres of the left vagus nerve via an electrode wrapped around the nerve in the cervical region. Though the exact mechanism of anti-epileptic action of VNS is not fully understood, it is likely to be related to effects on the thalamus and other limbic structures 2. VNS has been shown to result in median seizure reduction rates of approximately 45% at 12 months, with some evidence of continued improvement in seizure control over time 2-5. Greater seizure control is achieved on higher stimulation settings, but this also increases the risks of side effects from the therapy. The side effects are mainly stimulation-related, including dysphonia (up to 66% of patients), cough (45%), throat pain (28%), and headaches (24%). They are generally well-tolerated, tend to improve over time, and mostly resolve with decreased intensity of stimulation 3, 6.
VNS – Procedure
VNS therapy consists of a number of components including: (1) pulse generator, (2) electrode leads, (3) programming wand, and (4) handheld computer with installed software.
The pulse generator (Pulse Model 102 Generator) is an implantable, multi-programmable generator that is housed in a titanium case and powered by a single battery. It is responsible for delivering stimulating electric currents via a bipolar electrical lead (Model 302) to the vagus nerve (see Fig 1).
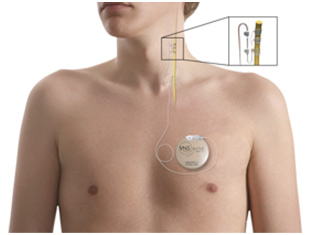
Figure 1: VNS therapy device 7
Once implanted, the pulse generator is programmed by the Model 250 Programming Software, which is loaded onto either a laptop or handheld computer dedicated only to programming the VNS Therapy System. A programming wand (NeuroCybernetic Prosthesis Programming Wand, Model 201) connected to the handheld computer via a cable serves as the interface with which to interrogate the pulse generator and modify stimulation parameters 6 (see Fig 2).
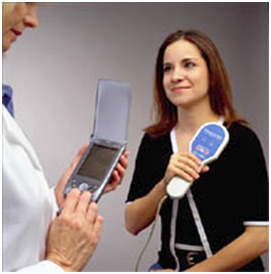
Figure 2: Programming of the VNS therapy device using the Programming Wand and handheld computer 8
In this case, the computer used was a Dell Axim X5 handheld computer, a member of Dell’s line of Windows Mobile-powered Pocket PC devices with a retail price starting at $279. As it has no intrinsic Internet connectivity, software updating is performed via compact flash 9.
Discussion
Increasingly, companies are using general-purpose mobile or handheld devices in the market to operate their medical devices. The versatility of generic mobile devices, as reflected by the number and diversity of mobile applications or “apps” available in the technology market 10, allows them to function as control tools for proprietary medical devices, given the proper software to connect to the medical devices. There are two main advantages to this approach, rather than creating custom-built control devices for new medical products.
Firstly, it reduces cost to the company. It is likely to be cheaper to reprogram an existing mobile device for the use of a specialist purpose such as VNS therapy than to design a new de-novo device. There is a wide range of low cost mobile devices in the market today with sufficient capabilities to be used in operating medical devices, such as the IPod Touch (RRP starting at $219), and various Android-based phones from companies such as HTC (One V $204, Desire $264), Samsung (Galaxy Ace 2 $240), Nokia (Lumia 800 $260), and Sony Ericson (Xperia $263) (www.shopbot.com.au).
More importantly, given clinician familiarity with current mobile technology especially smartphones 11, producing medical devices that can be operated by existing smartphones can increase the usability of the device and will allow more clinicians to adopt it into clinical practice. Many such medical devices are already emerging in the market. Health-monitoring devices that interface with smartphones, for instance, are gaining ground in the routine provision of healthcare. These include blood pressure monitors, blood sugar level (BSL) monitors 12, and foetal monitors 13. Other mobile-equivalents of traditional devices are also making inroads into medical practice including digital stethoscopes 14, mobile ultrasound probes 15, eye assessment tools 16, and mobile electro-cardiograms (ECGs) 17. The use of familiar mobile platforms to operate these medical devices provides clinicians with the ease of access to utilise these emerging technologies.
The disadvantages to this option, however, also need to be carefully considered. The use of reprogrammed mobile devices in medicine is dependent on a range of technical issues, such as software stability, compatibility, and device connectivity. For example, the handheld computer used in the VNS therapy failed once during the procedure due to software-related issues. While this did not result in significant adverse outcomes for either the surgery or the patient, the failure of a device during a higher risk or time-critical procedure can be of significant concern.
Another consideration is that there may be greater cost savings to the company if purpose-built computer devices are produced at scale, especially for more widely used medical products. For example, integrating and streamlining software and hardware production can be a cheaper option than acquiring the components separately. Prices of mobile devices in the market often include additional costs for marketing, advertising, postage and handling. Softwares that are developed for existing mobile platforms may require additional testing for device compatibility and stability, thus potentially increasing the production time and cost.
Connectivity
Connectivity is also another important issue to consider in the use of mobile technologies to operate medical devices.
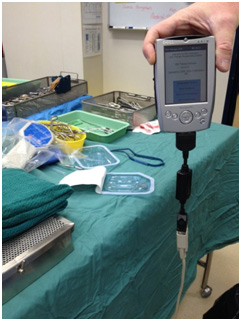
Figure 3: Cable connection between the Dell Axim X5 to the programming wand
The handheld computer in the VNS Therapy was connected to the programming wand via a cable. The obvious downside of this approach is that it adds bulk to the device, creates potentials for safety hazards, and also becomes potential points for connection failure (see Fig 3). The lack of Internet connectivity also makes it more difficult to perform software updates, and the collation of data for monitoring and auditing purposes is potentially more cumbersome.
The upside of the lack of connectivity is that there is greater device security. There is increasing security concerns of wireless medical devices in terms of data and patient safety. Recent safety issues have been raised around vulnerabilities in such devices, allowing them to be “hacked”, and even controlled remotely by those with sufficient technical skill and proficiency. This can prove disastrous when sensitive devices such as insulin pumps and cardiac pacemakers are involved 18, 19. The added benefits of having Internet connectivity obviously need to be balanced out with potential security risks.
Conclusion
In summary, there is an emerging market for medical devices operated by existing mobile devices. There are advantages to the healthcare sector in terms of familiarity with the mobile devices being used. Concurrently, there are also potential issues that need to be addressed in terms of technical performance and security risks.
References
1. Englot DJ, Chang EF, Auguste KI. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration, and seizure type. Neurosurgery Clinics of North America. 2011;22(4):443. 
2. Ben Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurology. 2002;1(8):477. 
3. Schachter SC. Vagus nerve stimulation therapy summary Five years after FDA approval. Neurology. 2002;59(6 suppl 4):S15-S29. 
4. Amar AP. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004;55(5):1086. 
5. Uthman B, Reichl A, Dean J, Eisenschenk S, Gilmore R, Reid S, et al. Effectiveness of vagus nerve stimulation in epilepsy patients A 12-year observation. Neurology. 2004;63(6):1124-6. 
6. Cyberonics. Physician’s Manual: VNS Therapy System. Houston, Texas: Cyberonics, Inc; 2010.
7. Cyberonics. VNS Therapy for Epilepsy Basics: How does VNS Therapy work? 2012 [cited 2012 Sep 20]; Available from: http://us.cyberonics.com/en/vns-therapy-for-epilepsy/patients-and-families/basics/how-does-vns-therapy-work
8. Cyberonics. VNS Therapy: Products. 2012 [cited 2012 Sep 20]; Available from: http://us.cyberonics.com/en/vns-therapy-for-epilepsy/healthcare-professionals/vns-therapy/about-products
9. Dell Axim X5 Basic and Advanced Pocket PCs. 2002 [cited 2012 Sep 25]; Available from: http://www.mobiletechreview.com/dell_axim_x5.htm
10. Brian M. Smartphone apps set to surpass the 1 million mark next week. 2011 [cited 2012 Nov 30]; Available from: http://thenextweb.com/mobile/2011/12/02/smartphone-apps-set-to-surpass-the-1-million-mark-next-week/?&_suid=1354274548732011648659314960241
11.Manhattan Research. 75 percent of U.S. Physicians own some form of Apple device. 2011 [cited 2012 Sep 20]; Available from:
12.Melanson D. Sanofi-Aventis debuts iBGStar blood glucose monitor for iPhone. 2010 [cited 2012 Sep 29]; Available from: http://www.engadget.com/2010/09/21/sanofi-aventis-debuts-ibgstar-blood-glucose-meter-for-iphone/
13.Ostrovsky G. The AirStrip OB(R) for wireless fetal heart rate monitoring. 2006 [cited 2012 Sep 29]; Available from: http://medgadget.com/2006/02/the_airstrip_ob_1.html
14. Ostrovsky G. Thinklabs iPhone app pairs up with electronic stethoscope. 2010 [cited 2012 Sep 29]; Available from: http://medgadget.com/2010/02/thinklabs_iphone_app_pairs_up_with
_electronic_stethoscope.html
15. Moore E. Smartphone ultrasound device hits market. 2011 [cited 2012 Sep 29]; Available from: http://news.cnet.com/8301-27083_3-20118706-247/smartphone-ultrasound-device-hits-market/
16. Bastawrous A, Leak C, Howard F, Kumar V. Validation of near eye tool for refractive assessment (NETRA) – Pilot study. Journal of Mobile Technology in Medicine. 2012;1(3):6-16. 
17. Nafziger B. New cell phone takes ECG readings. 2010 [cited 2012 Sep 29]; Available from: http://www.dotmed.com/news/story/13886
18. Leavitt N. Researchers fight to keep implanted medical devices safe from hackers. Computer. 2010;43(8):11-4. 
19. Robertson J. Hacker shows off lethal attack by controlling wireless medical device. 2012 [cited 2012 9 Sep]; Available from: http://go.bloomberg.com/tech-blog/2012-02-29-hacker-shows-off-lethal-attack-by-controlling-wireless-medical-device/
Read More




 The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 4S, published in December 2012. As media partners of the mHealth Summit 2012, Journal MTM is proud to present a special edition of the Journal presenting all the conference abstracts. We look forward to your article submissions.
The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 4S, published in December 2012. As media partners of the mHealth Summit 2012, Journal MTM is proud to present a special edition of the Journal presenting all the conference abstracts. We look forward to your article submissions.