Use of the WelTel mobile health intervention at a tuberculosis clinic in British Columbia: a pilot study
Mia L. van der Kop MSc1,2, Jasmina Memetovic, MSc3, Kirsten Smillie, MA4, Jesse Coleman, MA5, Jan Hajek, MD3, Natasha Van Borek MA4, Darlene Taylor, MSc4,6, Kadria Alasaly, MD4, James Johnston, MSc, MD4, Richard T. Lester, MD3,4, Fawziah Marra, PhD7
1University of British Columbia Centre for Disease Control, Vancouver, Canada; 2Department of Public Health Sciences, Karolinska Institutet, Stockholm, Sweden; 3Department of Medicine, University of British Columbia, Vancouver, Canada; 4Clinical Prevention Services, British Columbia Centre for Disease Control, Vancouver, Canada; 5Wits Reproductive Health & HIV Institute, Johannesburg, South Africa; 6School of Population and Public Health, University of British Columbia, Vancouver, Canada; 7Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, Canada
Corresponding Author: miavanderkop@gmail.com
Journal MTM 2:3:7–14, 2013
Background: Successful treatment of latent tuberculosis infection (LTBI) is critical to reduce the impact of tuberculosis (TB). The purpose of this study was to determine the feasibility of adopting the WelTel text-messaging intervention, proven to be effective in improving HIV treatment adherence, for use in LTBI care.
Aims: (1) Determine prevalence of mobile phone ownership, text-message use, and patient attitudes towards receiving text messages from the clinic. (2) Determine the technological feasibility of the WelTel intervention, and (3) patient and healthcare provider acceptability of the service.
Methods: We conducted a cross-sectional descriptive survey of patients attending a provincial TB clinic and a pilot study in which patients initiating treatment for LTBI received the intervention for 12 weeks.
Results: Clinic survey: Of 82 participants who completed the survey between September and December 2011, 68 owned a mobile phone and 58 used text messaging weekly. Participants were receptive to receiving text-message communication from the clinic (n = 80). Pilot intervention study: Of 16 patients who received the intervention, 14 completed the study. Using software to deliver the intervention was feasible. The greatest participant-perceived benefit was that it enabled them to report side-effects quickly (n = 6); the greatest participant-perceived barrier was cost (n = 3).
Conclusion: The majority of patients in this study population had the means to communicate with their healthcare providers via text-messaging and were receptive to doing so. The intervention was well-received by participants and the healthcare provider. A randomized controlled trial is underway to determine the intervention’s effectiveness to increase LTBI treatment adherence.
INTRODUCTION
Successful treatment of latent tuberculosis infection (LTBI) is critical to reduce the impact of TB; however, in North America, fewer than half of individuals starting LTBI treatment complete therapy.1, 2 Existing TB treatment adherence interventions have not yet proven consistently successful,3 and while innovative interventions using text-messaging have been developed to improve adherence (e.g. SIMPill®, X out TB, etc),4 rigorous evaluations of their effectiveness are pending. Evidence has shown, however, that weekly text messages can improve treatment adherence and health outcomes in individuals with HIV.5 One of these evidence-based interventions is WelTel, a service involving weekly text-message “check-ins” with patients, with clinician follow-up for patients who indicate they have a problem.6 For example, every Monday morning, patients are sent a text message asking how they are doing. They are instructed to respond within 48 hours either that they are well or that they have an issue they would like to discuss. A clinician calls those who respond with an issue or who do not respond; those who indicate they are well are simply sent a message again the following week. The aim of this pilot study was to determine the feasibility of adopting the WelTel intervention, originally developed and tested in Kenya, for use in the context of LTBI care in British Columbia, Canada. We conducted a cross-sectional survey among TB clinic patients to: i) estimate the prevalence of cell phone ownership and use of text-messaging; and ii) assess patient attitudes towards receiving text messages from the clinic (Figure 1). As the formative phase of a randomized controlled trial (RCT), we then implemented the intervention in a group of LTBI patients to determine the technological feasibility of the intervention, and patient and healthcare provider acceptability of the service.
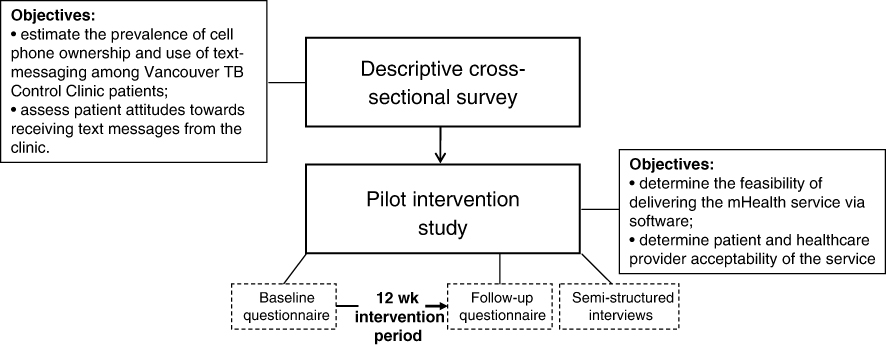
Figure 1: Conceptual overview of the study
METHODS
Clinic survey
Between September and December 2011, a descriptive cross-sectional survey was undertaken at the Vancouver provincial TB clinic. We did not formally calculate sample size; however, we targeted obtaining 100 completed surveys. Patients were eligible to participate if they were >18 years of age; initiating treatment for LTBI or active TB; and willing to provide informed consent. The clinic nurse or pharmacist invited patients to participate in the survey when they picked up their medication at the pharmacy; recruitment was conducted on a continuous basis. The self-administered questionnaire focussed on demographics, mobile phone ownership and use, and had Likert-type questions on attitudes towards receiving text messages from the clinic. Participants were not reimbursed for participation. Data were entered and analysed using descriptive statistics (number and percentage) in SPSS version 14.0 (SPSS Inc., Chicago, USA). Data cleaning procedures included range and consistency checks.
Pilot intervention study
A prospective pilot study was conducted at the same clinic between April and October 2011. An enrolment target was set at between 20 and 50 participants. The clinical research coordinator continuously recruited patients who were: initiating LTBI treatment (either isoniazid [INH] for nine months or rifampicin [RIF] for four months); >18 years of age; had access to a mobile phone; and able to communicate via text messaging in English or had somebody who could read and respond to the text messages on their behalf. Figure 2 provides information on the flow of participants through this aspect of the study. After providing informed consent, participants completed a clinician-administered baseline questionnaire on demographic factors, cell phone use, and communication with healthcare providers. In addition to standard LTBI care, all participants received the WelTel intervention for 12 weeks. Participants were informed that the WelTel service does not replace existing clinical care or emergency services. An automated system was used to send text messages from a laptop computer at the clinic. The computer and programs were password protected and all study databases were encrypted to protect participant confidentiality. The weekly text message sent to participants stated “How are you doing?” (Figure 3) Participants were asked to reply within 48 hours indicating whether they were either “OK” or “Not OK.” Non-response and indications of a problem were followed-up by the clinician with a phone call. At the end of the 12-week period, participants completed a follow-up questionnaire administered by the research coordinator. Pilot study participants were provided with $20 CAD for participation; they were not reimbursed for costs associated with responding to the weekly messages. Questionnaire and text-message data were entered and analysed in SPSS version 14 (SPSS Inc., Chicago, USA). A chi-square test was used to determine whether the proportion of non-response changed over time (weeks 1–4 v. 5–8 v. 9–12).
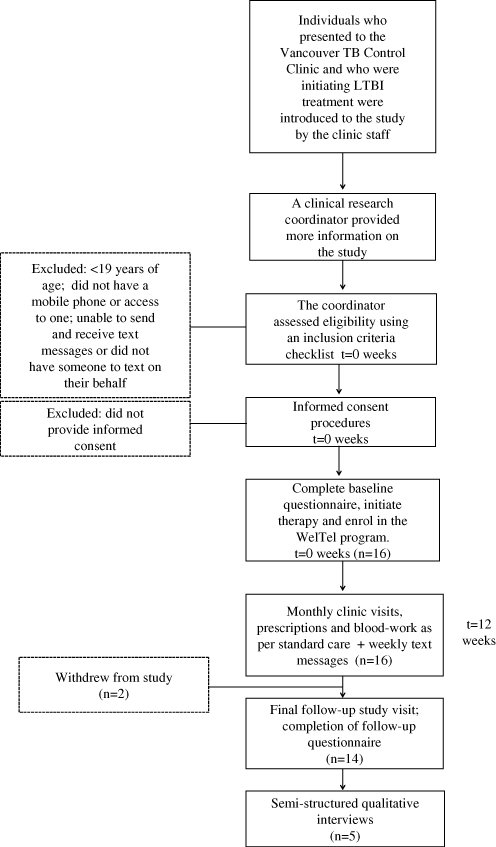
Figure 2: Participant flow through the pilot intervention study
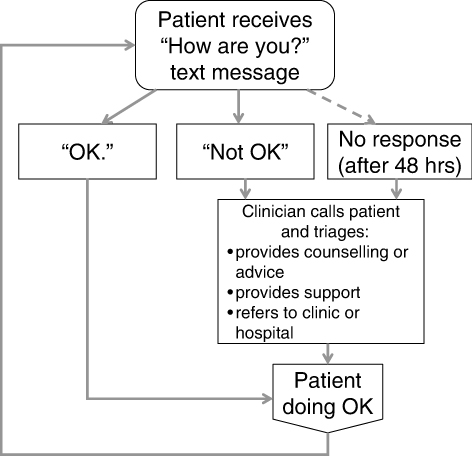
Figure 3: The WelTel Intervention
At study end, a trained qualitative researcher conducted semi-structured interviews with the clinical research coordinator participating in the program and five participants who were selected through convenience sampling. Participants’ perspectives about their experiences with the intervention were explored, and recommendations to improve the intervention were solicited. Interviews were conducted in English, 30–60 minutes in length, and recorded and transcribed verbatim. A qualitative researcher analysed the data using an interpretive descriptive approach, and identified concepts and themes related to participants’ experiences with the WelTel intervention.
Ethics statement
Ethical approval for the survey (H11-02096) and pilot study (H11-02889) was obtained from the University of British Columbia’s Behavioural Research Ethics Board and Clinical Research Ethics Board respectively.
RESULTS
Clinic survey
Between September and December 2011, 82 patients completed the questionnaire. Data on the response rate was not collected. Most participants were female (n=50), had LTBI (n=67), and were born outside of Canada (n=73) (Table 1). The median age was 41 years (range 19–84). Over 80% of participants owned a mobile phone (n=68). Use of text-messaging was prevalent among participants: 72% of all participants had used text-messaging (n=59), and approximately 70% of participants sent and received at least one text message a week. Over half (61%) of participants had never communicated with a health care provider using their mobile phone. The majority of participants stated English as their preferred language to text (n=68).

Table 1: Cross-sectional survey participants: demographic and clinical characteristics, cell phone ownership and prevalence of text-messaging
On a Likert-type scale of one to five (with 1=not at all and 5=very much so), the median response to a question on how comfortable people would be receiving general TB information via text was 3.5. Participants were receptive to receiving information specific to their TB treatment, with a median response of 4 (total n=109). About half of participants (49%) would prefer not to include the words “TB” or “tuberculosis” in text messages sent from the clinic to their phones (n=40). Responses varied in how frequently participants would like to receive treatment-related messages from the clinic: 18% indicated daily (n=15); 22% weekly (n=18); 23% twice a month (n=19); and 34% once a month (n=28). Less than 3% of participants thought that text messages relating to treatment were unnecessary (n=2).
Pilot study
Study population
Between April and July 2011, 16 patients initiating either INH (n=14) or RIF (n=2) were recruited. The median age of participants was 47 (range 21–82); 56% of participants (n=9) were female. Most participants were born outside of Canada (n=13) and most comfortable communicating in English (n=13), rather than another language. Only one participant did not own a cell phone (the participant received the service on his son’s phone; his son had agreed to text on the participant’s behalf). At baseline, 75% of participants had previously engaged in text-messaging (n=12). Of the 16 participants, 14 completed follow-up; one participant withdrew at week 8 (moved out of country) and another withdrew at week 9 for unknown reasons.
Text messages sent and received
Over the 12-week study period, 180 messages were sent to participants (Table 2). In error, five messages that should have been sent were not. Of responses, 76% indicated the participant was “OK” (n=137), and 19% were instances of non-response (n=35). The primary reason for non-response was technological: either the participant did not receive the text message, or the participant indicated they had sent a text message and the SMS gateway did not record it (n=13). Other reasons for participant non-response are listed in Table 2. Non-response increased from 9% (6/64) in the first 4-weeks of participation to 22% (14/64) and 29% (15/52) in subsequent four-week periods respectively (p=0.010). Apart from one patient who had been taken off their medication and wished to resume, all indications of a ‘problem’ (n=8 from 4 participants) were due to medication side-effects.
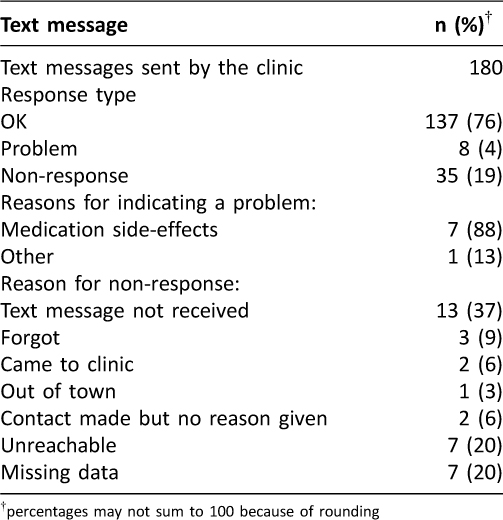
Table 2: Pilot study among LTBI patients: Text messages sent and received during the 12-week intervention period
Follow-up
Of 14 participants who finished the 12-week program, 13 completed follow-up questionnaires. One of the participants who had withdrawn completed a final questionnaire. Half of the participants had some difficulty receiving the text messages (n=7/14) and 36% had difficulty sending them (n=5/14). Barriers to using the service cited by participants included expensive network charges (n=3), being in an area with no network coverage (n=2), technological difficulties (n=2), dependency on others to send and receive messages (n=1), and not being very good at texting (n=1). Of those who cited barriers, half stated that the barrier would not prevent them from communicating with the clinic via cell phone in the future. Overall, participants were positive about the service: 10 found the service helpful or very helpful; 4 had neutral feelings; and none felt that the service was a nuisance. Most participants felt that the frequency of the messaging was ‘just right’ (n=12), with one participant indicating that messages were too frequent, and another that they were not frequent enough. The greatest participant-perceived benefits were that it enabled them to report side effects quickly (n=6), reminded them to take their medication (n=4), and imparted a feeling that their healthcare providers cared about them (n=2). The majority of participants indicated they would like the program to continue (n=11) and 86% would recommend it to a friend (n=12).
Technological feasibility
Text messages were sent from a laptop with a data stick, using FrontlineSMS (Version 1) software. FrontlineSMS was chosen because it was free and quick to implement. After initiating the pilot study, limitations of FrontlineSMS were identified e.g. patients had not received messages despite the system indicating that they had been sent. At the end of the sixth week, we switched to a custom-built text-message gateway and database system, which was used for the remainder of the study. The custom-built software system sent the text messages at the same time every week, sorted responses to identify when patients were having problems and required follow-up, and automatically identified non-responses. After implementing the custom-built software, there were no further technical issues with sending or receiving messages.
Semi-structured interviews with participants and the clinical research coordinator
Clinician’s experienceThe clinical research coordinator was generally positive about the WelTel intervention, stating that the weekly check-ins allowed for faster detection of adverse events, and that increased communication made patients feel better supported by their healthcare team. Additional patient benefits cited included regular opportunities to ask questions, having a “listening ear”, and receiving support related to stigma associated with TB. The clinical research coordinator thought that text messaging may be of particular benefit to patients who have issues with treatment (adherence or side-effects) or are prone to liver toxicity. Although there were some technical difficulties and an increased workload (additional paperwork and clinic time) initially, the increased interactions with patients made it worthwhile. The coordinator also felt that the WelTel service would not pose a significant administrative burden if the computer platform sending the messages was functioning properly, and was in favour of further research to determine the clinical effectiveness of the intervention.
Patients’ experiencesParticipants stated that receiving the text messages made them feel supported by their healthcare provider, as though someone “cared”, and that it was reassuring to be able to report side effects immediately. Convenience was another identified benefit: participants did not need to make an appointment if they had an issue, and could text at a time that suited them. Although participants felt there were many benefits to the intervention, they did not feel that text messaging could entirely replace face-to-face contact or phone calls. Barriers to using the service included not knowing how to text and having little interest in learning, with a consequent reliance on others to respond for them. One family member who received the messages for her father noted that they were a good reminder for her to “check-in” with her father on how he was coping with medications. A participant who was out of cell phone range for a week noted cell phone reception as a barrier to responding.
Participants’ suggestionsA few (n=3) participants thought that the weekly text messages should include appointment reminders. One participant noted that a next-of-kin who was involved with maintaining clinic appointments should also receive the weekly message, and another suggested that email may be more suitable for those who don’t know how to text. Most participants felt that the service should be publicly funded.
DISCUSSION
This study found that the majority of TB clinic patients in our study population owned a mobile phone, used text-messaging regularly, and were receptive to receiving text message communication from the clinic. Patients in the pilot study found the intervention helpful, in particular as a general reminder to take their medication and to report side effects promptly. In both questionnaires and semi-structured interviews participants noted “feeling like somebody cares”. The WelTel intervention may positively affect some of the factors associated with treatment completion rates, including availability of expertise, the healthcare provider-patient relationship, and quality of healthcare delivery.7 Although most participants would have liked the program to continue, it was not without its barriers, including high costs associated with network charges and for some, being dependent on somebody to text on their behalf (if they did not own their own mobile phone or could not text). The clinician valued the program for the additional support that it provided to patients and the opportunity it gave them to report side-effects, which could then be followed-up in a timely manner. From the clinician’s perspective, the largest barrier to the WelTel service was the technological difficulties related to the platform.
This was the first study involving the WelTel intervention that used a prototype technological platform to deliver and receive patient-provider text messages. In the original WelTel Kenya1 trial, the bulk-messaging feature on the clinician’s mobile phone was used and all messages were manually recorded.6 The objective of using a technological platform to deliver the service was to decrease clinician workload. For the first few weeks of the pilot study, the platform was fraught with difficulties: not all patients were receiving the texts, and the platform was not recording all incoming text messages. In these instances, the clinician manually followed up by telephone, increasing workload associated with managing the service. Once the technological system was working properly, the clinician no longer felt that the WelTel-related workload was burdensome.
Historically, the focus of knowledge and innovation transfer has been uni-directional, from North to South; increasingly however, the diffusion of innovation in healthcare is bi-directional. One of the strengths of this study is that it was the first step in determining whether the WelTel intervention, originally found effective at improving adherence to HIV treatment in Kenya,6 is a feasible and acceptable strategy for use in a North American setting among LTBI patients. Methodologically, one of the study’s strengths is that responses to questions in the initial survey and pilot study questionnaires were highly complete. Limitations of both aspects of this investigation include potential selection bias. Clinicians incorporated research activities, including recruitment, into routine care. Consequently, information on response rates to the survey was not collected due to time constraints, and data were not collected on those who declined participation. As a result, we are unable to assess the extent to which non-response may have affected the results.
While the pilot study fulfilled its objectives of providing an initial examination of patient and healthcare provider acceptability of the intervention, to stay within funding parameters, it was of shorter duration than a full course of LTBI treatment. As a result, we were unable to determine the intervention’s sustainability over the entire LTBI treatment period. Another limitation of the study, its small sample size, was compounded as the initial targeted number of participants was not met due to slower than expected recruitment, likely related to a competing trial at the study site. The intervention’s longer-term sustainability and effectiveness to improve LTBI treatment completion rates are currently being investigated in a follow-up RCT (ClinicalTrials.gov identifier: NCT01549457).
Consistent with a recent North American study reported by Person et al, the majority of TB clinic attendees had the means to communicate with their healthcare providers via text-messaging.8 Our study also confirmed the finding that patients are more receptive to receiving medication ‘reminders’ than general medical education via text-message.8 Non-response to the outgoing text messages approximated that of the original WelTelKenya1 trial,9 and similarly, a low proportion of patients responded with a problem. Other mHealth interventions to improve adherence to TB treatment are also being explored. These include the SIMpill, a medication container that delivers a text to a central server when opened, and the Adhere.IO system, an incentive-based system in which patients text a code that is revealed when they urinate on a filter paper and TB drug metabolites are detected.10 Unlike these other interventions, WelTel does not require distributing additional devices to patients to report adherence, and it involves regular and when required, personalized communication with the clinic on a broad range of issues. Potential advantages of the WelTel service include its simplicity, its use of technology already in the hands of most patients (a standard mobile phone), and that the provision of a regular ‘check-in’ may be perceived as more supportive by patients than interventions specifically designed to ‘monitor’ treatment adherence.
CONCLUSION
Patients in our study population had the means to communicate with their healthcare providers through text-messaging and were receptive to doing so. After overcoming initial difficulties with the software platform, implementing the WelTel service in a group of participants was technologically feasible and well-received by both the healthcare provider and patients. An RCT, currently underway, will determine if in addition to being feasible and acceptable, the intervention is effective in improving treatment adherence among LTBI patients.
ACKNOWLEDGEMENTS
We thank the TB clinic staff and patients who participated in this study.
AUTHORS’ CONTRUBUTIONS:
RTL, FM, DT, JH, and JC conceived and designed the study. KA, NVB, DT, FM, and JC were substantially involved in the acquisition of data. MVDK, KS, JM, FM, JJ, JH, KA and NVB analysed and interpreted the data. MVDK, JM, KS and JC drafted the article. All authors revised the manuscript for critical intellectual content and approved the submitted version.
FUNDING
No support was received to conduct the cross-sectional survey. The pilot study was funded by the British Columbia Centre for Disease Control (BCCDC) Communal Fund (grant reference no. 20R65527). MvdK is supported by a Canadian Institutes of Health Research Doctoral Award – Doctoral Foreign Study Award (October 2012), offered in partnership with the CIHR Strategy for Patient-Oriented Research and the CIHR HIV/AIDS Research Initiative.
CONFLICTS OF INTEREST
RTL is the founder of WelTel, a non-profit non-governmental mHealth organization with the goal of scaling up evidence-based mHealth solutions; he has no financial stake in or salary from the organization. For the remaining authors, no conflicts of interest were declared.
DISCLOSURE
The corresponding author is the recipient of a Canadian Institutes of Health Research Doctoral Award.
References
1. Horsburgh CR Jr., Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010 Feb;137(2):401–9. ![]()
2. Horsburgh CR Jr., Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med. 2011 Apr 14;364(15):1441–8. ![]()
3. Hirsch-Moverman Y, Daftary A, Franks J, et al. Adherence to treatment for latent tuberculosis infection: systematic review of studies in the US and Canada. Int J Tuberc Lung Dis. 2008 Nov;12(11):1235–54.
4. Barclay E. Text messages could hasten tuberculosis drug compliance. Lancet. 2009 Jan 3;373(9657):15–6. ![]()
5. Horvath T, Azman H, Kennedy GE, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;3:CD009756. ![]()
6. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45. ![]()
7. Sabateé E. Adherence to long-term therapies. Geneva: World Health Organization; 2003.
8. Person AK, Blain ML, Jiang H, et al. Text messaging for enhancement of testing and treatment for tuberculosis, human immunodeficiency virus, and syphilis: a survey of attitudes toward cellular phones and healthcare. Telemed J E Health. 2011 Apr;17(3):189–95. ![]()
9. van der Kop ML, Karanja S, Thabane L, et al. Indepth analysis of patient-clinician cell phone communication during the WelTel Kenya1 antiretroviral adherence trial. PLoS ONE. 2012;7(9):25. ![]()
10. Denkinger CM, Grenier J, Stratis AK, et al. Mobile health to improve tuberculosis care and control: a call worth making [Review article]. Int J Tuberc Lung Dis 2013;17(6):719–27. ![]()

