Development and evaluation of an iOS vancomycin clinical decision support software application
William H. Spires, Pharm.D., M.B.A.1, Amber Wesner, Pharm. D., BCPS1, Robert S. Kidd, Pharm.D., Ph.D.1
1Department of Pharmacy Practice (AR Wesner) and Department of Biopharmaceutical Sciences (RS Kidd) Shenandoah University, Winchester, Virginia. From the Bernard J. Dunn School of Pharmacy (WH Spires) Shenandoah University, Winchester, Virginia
Corresponding Author: rkidd@su.edu
Journal MTM 4:2:2–11, 2015
Background: Due to changes in treatment guidelines, traditional pharmacokinetic based dosing and existing vancomycin nomograms may not predict doses adequate for reaching the increased trough concentration goals. A novel nomogram was created to achieve the updated goals, and the nomogram was previously validated at our institution. For this study, the nomogram was converted into an iOS vancomycin clinical decision support software (CDSS) application.
Aims: This study was designed to determine if the use of the iOS vancomycin CDSS application decreases the time pharmacists spend dosing vancomycin thus resulting in a cost savings to the health-system.
Methods: Enrolled patients’ dosing regimens were determined using the newly developed iOS vancomycin CDSS application. Survey data was used to assess pharmacists’ impressions of the iOS vancomycin CDSS application and the time required to determine a personalized vancomycin dosing regimen among several different dosing methods. The accuracy of the iOS vancomycin CDSS application was also evaluated and compared to data from the original paper version of the dosing nomogram.
Results: A total of 367 patients were dosed using the iOS vancomycin CDSS application, and 146 of these patients met all inclusion criteria for accuracy analysis. The mean time savings of using the iOS vancomycin CDSS application versus traditional pharmacokinetic calculations was 10.3 minutes per consult (p = 0.014). The iOS vancomycin CDSS application was estimated to save a total $13,617 per year compared to using traditional vancomycin pharmacokinetic calculations at our institution. There was no statistical difference in therapeutic trough concentration frequencies between the paper nomogram and the iOS vancomycin CDSS application (p =0.877).
Conclusion: An iOS vancomycin CDSS application saved pharmacist’s time, established a moderate cost-savings, was well accepted, and accurately predicted appropriate vancomycin dosing regimens. This iOS CDSS drug dosing application was only used for vancomycin, and therefore the time and costs savings could increase substantially as more drugs are incorporated into the CDSS application.
Introduction
Vancomycin, a glycopeptide antibiotic, is commonly used to treat a variety of gram-positive infections in the health-system setting.1 However, because of potential adverse drug events related to supra-therapeutic trough concentrations, the potential for treatment failure, and development of bacterial resistance due to sub-therapeutic trough concentrations, vancomycin concentrations are monitored regularly to ensure appropriate trough levels are achieved. Target trough concentrations were previously considered therapeutic when a 5–15 mcg/mL concentration was achieved.2 However, due to bacterial resistance, the American Society of Health-System Pharmacists/Infectious Diseases Society of America/Society of Infectious Diseases Pharmacists published a consensus statement in 2009 updating the guidelines for appropriate vancomycin trough concentrations.3 These new guidelines recommended trough concentrations remain above 10 mcg/mL in order to prevent treatment failure and resistance, and those patients being treated for severe infections such as bacteremia, endocarditis, meningitis, MRSA pneumonia, osteomyelitis or sepsis, should maintain trough concentrations between 15–20 mcg/mL to ensure adequate treatment.3 The elevated vancomycin trough concentrations are due to the emergence of vancomycin-resistant Staphylococcus aureus which can be classified as vancomycin–resistant Staphylococcus aureus (VRSA) or vancomycin-intermediate Staphylococcus aureus (VISA), based on the reported minimum inhibitory concentrations (MICs). There is also a unique hetero-resistant Staphylococcus aureus (hVISA) that shows inducible resistance after exposure to vancomycin.3,4
Due to these changes in vancomycin trough concentration goals, existing vancomycin nomograms may not predict doses adequate for reaching these goals. For example, nomograms such as the Matzke, Lake and Peterson, Rotschafer, Nielson, and Moellering were not designed to achieve trough levels greater 15 mcg/mL and most were actually designed to reach trough levels of 10 mcg/mL or less.5–9
There have been several dosing algorithms designed to achieve these higher vancomycin trough levels.10–12 The nomogram developed by Kullar et al. was designed to reach target troughs between 15 and 20 mcg/mL and was effective in 58% of the patients studied, but had a significant number of exclusion limiting its application.10 The dosing protocol developed by Devabhakthumi et al. retrospectively showed an increase in the number of patients that were initiated on an appropriate vancomycin dose, defined by the authors as 15 mg/kg/dose, but there was no improvement in the percentage of patients achieving appropriate trough concentration per the new protocol.11 The nomogram developed by Thalakada et al. was effective in 56% of patients, but it is only designed to address the dosing interval and to achieve trough concentration levels between 15 and 20 mcg/mL. 12
Two retrospective studies were completed at our institution to evaluate novel vancomycin dosing nomograms designed to achieve the higher vancomycin trough concentration goals. A prospective, randomized, open-label trial was conducted with a final, single nomogram (Figure 1) and found that upon initial dosing the nomogram achieved target trough concentrations more frequently than using traditional pharmacokinetic calculations (44% vs 33%, respectively, p = 0.014).13 The results of that study validated the novel vancomycin dosing nomogram as an effective dosing tool, and it became the standard dosing tool for vancomycin at our institution.
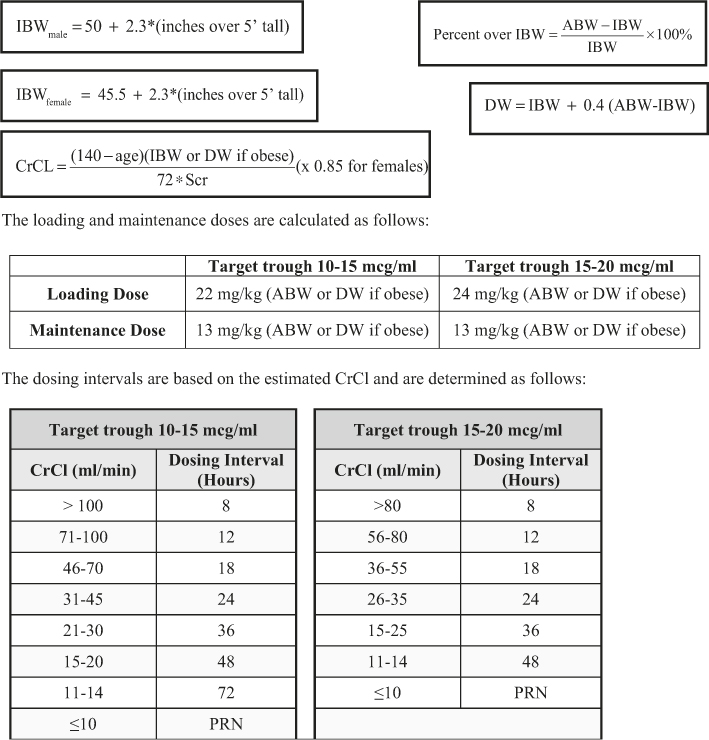
Figure 1: Vancomycin Nomogram
Comparing this nomogram to the one developed by Kullar el al., it can be used to achieve trough levels of 10–15 mcg/mL or 15–20 mcg/mL depending on the patient’s indication.10 The nomogram also included many patient populations that were excluded from Kullar et al. For example, the nomogram includes patients up to 120 kg, does not have an upper creatinine clearance cutoff for excluding patients, and critically ill patients are also included. Our nomogram has some similarity to the one developed by Devabhakthumi el al. because they both utilize weight to determine the vancomycin dose and creatinine clearance to determine the dosing interval. However, there were several differences between the two nomograms. The nomogram developed by Devabhakthumi et al. did not differentiate between obese and non-obese patients, did not separate trough goals, and if the patient’s trough fell between the range of 10–20 mcg/mL they were considered at goal despite individual indications. Our nomogram bases the trough concentration on indication, 10–15 mcg/mL or 15–20 mcg/mL, yet our results were comparable to those in the Devabhakthumi et al.11 Our accuracy was lower than determined in the study done by Kullar et al., 44% versus 58% respectively, which may be due in part to the fact that the patients included in the Kullar et al. study were relatively stable.10 This may be the case for the Thalakada et al. nomogram as well. Additionally, it should be noted that in our study, rounding was not done in order to provide a more conservative estimate of the nomogram’s accuracy. Absolute limits of 10.0–15.0 and 15.0–20.0 mcg/mL were used.13 Therefore, a trough concentration of 14.9 mcg/mL was not considered to be therapeutic if the trough concentration goal was 15–20 mcg/mL, based on the patient’s indication. As a result of the effectiveness of this newly developed nomogram, it was adopted as the preferred vancomycin dosing method at our institution.
Since technology has had a significant, positive impact throughout pharmacy and healthcare in general, further study into an electronic version of the nomogram was pursued to enhance ease of use and accessibility of the vancomycin nomogram for our clinicians. One major technology that has penetrated health-systems are iOS device applications.14,15 Using Apple’s application developer’s platform, Xcode, the paper nomogram was converted into an iOS device application. The development of the iOS vancomycin CDSS application allowed the vancomycin dosing nomogram to be automated and used on any iOS device throughout the institution. By automating the vancomycin dosing nomogram, we aimed to provide an accurate and more efficient vancomycin dosing tool for healthcare providers.
The primary objective of this study was to determine if the use of the iOS vancomycin CDSS application decreased the time pharmacists spend dosing vancomycin patients and therefore results in an overall time and cost savings. Secondary objectives of the study were to assess pharmacists’ impressions of the iOS vancomycin CDSS application and to compare the accuracy of the iOS vancomycin CDSS application to the paper based nomogram in achieving vancomycin target trough concentrations.
Methods
This prospective trial was conducted at a 454-bed community hospital on patients newly initiated on vancomycin therapy. Patients were included if they were less than or equal to 120 kg, greater than eighteen years of age, and had a new order for vancomycin where pharmacy was consulted to dose and monitor therapy. Exclusion criteria included: pregnancy, age less than eighteen years of age, weight greater than 120 kg, a serum creatinine increase of 50% or more from baseline during the patient’s course of vancomycin therapy, end stage renal disease requiring dialysis or any renal replacement therapy, and patients with anasarca. This study was approved and compliant with Shenandoah University’s and Winchester Medical Center’s Institutional Review Boards and adhered to the pharmacy departments approved vancomycin dosing policy.
The iOS vancomycin CDSS application was developed in-house using Xcode, Apple’s application developer’s software. An iOS developer’s account was established, and the application was “side loaded” onto several iPad Minis obtained by monies generated through an internal grant. The iPad minis were not only used for vancomycin dosing, but other pharmacy resources were loaded and utilized by the pharmacists. Therefore, the cost of the iPad Mini’s were not considered a direct expense of the study. The coding algorithms use standard if/else and case based statements, and the final results automate the paper nomogram calculations and look-up tables. The user inputs the patient’s age, height, weight, serum creatinine, target trough concentration range based on the patient’s indication, and gender via “picker wheels” and buttons as shown in Figure 2. After the “Accept Disclaimer” button is pressed, the program takes the user inputs and calculates the ideal body weight, percent over ideal body weight, dosing weight and creatinine clearance. From these calculations and the desired trough concentrations, the loading dose, maintenance and dosing intervals are determined and displayed as shown in Figure 3.
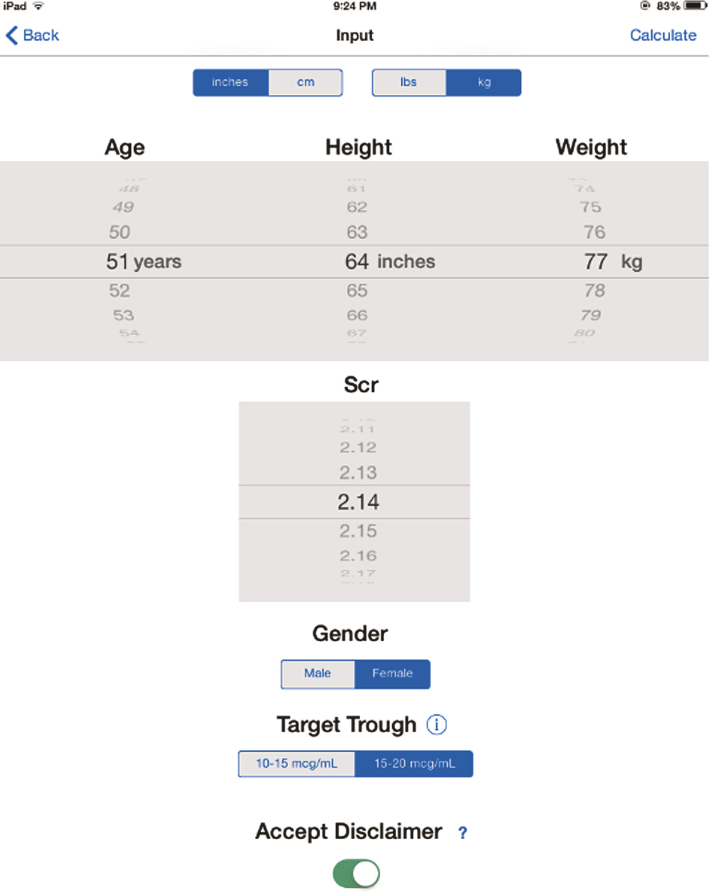
Figure 2: Screenshot of the iOS Vancomycin CDSS Application Input Screen

Figure 3: Screenshot of the iOS Vancomycin CDSS Application Results Screen
Pharmacists were educated on how to screen patients for inclusion in the study and how to use the iOS vancomycin CDSS application. The paper-based vancomycin nomogram is the standard dosing protocol at our institution, so all patients eligible for using the paper-based nomogram were eligible for the iOS vancomycin CDSS application during the study which constituted the study group. Pharmacists consulted to dose and monitor a patient’s vancomycin therapy determined the patient’s eligibility for inclusion and were encouraged to use the iOS vancomycin CDSS application. If a patient was eligible, the pertinent patient information was entered into the iOS vancomycin CDSS application, all applicable data was recorded on a hospital approved vancomycin consult form, and a colored sticker was placed on the consult form indicating that the pharmacist had used the iOS vancomycin CDSS application. Information required to use the iOS vancomycin CDSS application includes: patient’s age, height, weight, serum creatinine, gender, and target trough goal. An image of the iOS vancomycin CDSS application input screen and results screen is shown in Figures 2 and 3.
A single investigator educated all pharmacists. In-services on how to use the iOS vancomycin CDSS application as well as user’s guides were provided for all pharmacists to limit inter-clinician variability. Six iPad Minis were utilized and the iOS vancomycin CDSS application was loaded on each one. The iPad Minis were then strategically placed to optimize pharmacists’ access. The iPads were located in the main pharmacy and in each pharmacy satellite located throughout the hospital. Pharmacists that had personal iOS devices were also allowed to upload the application on their own device.
A questionnaire was used to evaluate user satisfaction and document any time-savings with the use of the iOS vancomycin CDSS application. The questionnaire consisted of seven statements covering different system attributes. Users were asked to rate their level of agreement to the statement using a five-point Likert scale with “1” corresponded to “strongly disagree” and “5” corresponded to “strongly agree.” The survey tool was designed to measure the users’ opinions of several key qualities including ease of use, impact on work efficiency, time savings realized, overall user satisfaction, reliability of the device, and device usage. An open text area was also included in the survey to assess any additional comments or suggestions from the users that were not covered by the other questions. Surveys were distributed at the conclusion of the study. The surveys were optional and anonymous, but all pharmacists were encouraged to complete the survey. The survey used during this study is shown in Figure 4.

Figure 4: Pharmacist’s Survey
For patients to have been included in the final accuracy analysis they must have received three identical doses of vancomycin, had a trough level drawn at steady state, which was defined as being just prior to the fourth or subsequent doses, and the iOS vancomycin CDSS application had to be used correctly. Correct use of the iOS vancomycin CDSS application was defined as the pharmacist using the dose and dosing interval derived by the iOS vancomycin CDSS application without modification. Vancomycin doses were capped at a total daily dose of 6,000 mg per day and 2,000 mg per dose based on our institution’s practice standards. Trough concentrations could have been drawn prior to steady state, if believed clinically necessary, but any changes to the dosing regimen based on this trough concentration resulted in the exclusion of the data from analysis. Extrapolated vancomycin troughs were calculated for all measured troughs used in the final data analysis. An extrapolated trough was defined as the vancomycin trough measurement just prior to the next vancomycin dose due. Extrapolated troughs were calculated out to one decimal place and had to lie within the absolute limits of 10.0-15.0 or 15.0-20.0 mcg/mL depending on the trough goal classification.
Data were analyzed with SPSS (SPSS statistics, version 22) and Microsoft® Excel® for Mac 2011 (version 14.3.6). Average time per dosing method (i.e. dosing done by the iOS vancomycin CDSS application, by the paper nomogram and by traditional pharmacokinetic calculations) was determined by survey data (Table 1) and compared with a one-way ANOVA and Tukey’s post hoc test. The cost savings realized using the iOS vancomycin CDSS application compared to traditional pharmacokinetic dosing was calculated based on the following formula: (Average time per dosing method)*(1500)*(pharmacist salary/minute). The value of 1500 is the average annual number of vancomycin dosing consults performed at this institution over the past three years. A pharmacist’s annual salary of $110,000 or $0.8814/min was used for the calculation. The value of $0.8814/min reflects a typical 40 hours per week and 52 weeks per year. The secondary objective, determining the accuracy of the iOS vancomycin CDSS application compared to the paper nomogram, was evaluated using the Chi-square test and included the results of the previous study as the control.4 A p-value less than 0.05 was considered statistically significant.
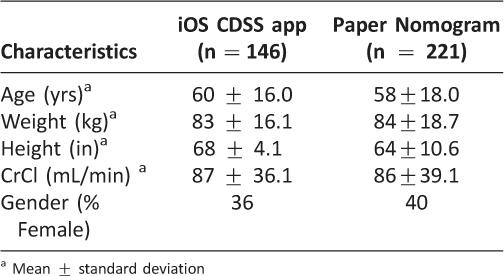
Table 1: Characteristics of Study Subjects
Results and Discussion
Results
Data collection occurred from October 10, 2013 through April 31, 2014. Four hundred twenty-one patients were reviewed for this study, 367 were dosed using the iOS vancomycin CDSS application, and 221 of these patients were withdrawn for accuracy analysis. Reasons for withdrawal included: discontinuation of vancomycin before trough levels were drawn, patient’s serum creatinine varied greater than 50% during therapy, inappropriate trough timing, patients being outside of the verified nomogram weight range, and patients on hemodialysis. One hundred forty six patients were included in the final accuracy analysis as shown in Figure 5. Patient and pharmacist’s demographic data are presented in Table 1 and 2, respectively. The most common types of infections noted were pneumonia, cellulitis, sepsis, skin/skin structure infection, osteomyelitis and urinary tract infections.

Figure 5: Vancomycin Consults Included in Study

Table 2: Pharmacists’ Demographics
A total of 15 surveys were completed resulting in a 56% response rate. The average time savings from using the iOS vancomycin CDSS application versus using traditional vancomycin pharmacokinetic calculations was 10.3 minutes per consult as shown in Table 3 (p = 0.014). This shortened time to complete vancomycin dosing consults resulted in an overall cost-savings of $13,617 per year by using the iOS vancomycin CDSS application. Pharmacists’ impressions of using the iOS vancomycin CDSS application are presented in Table 4. Pharmacists reported that they were very comfortable using the CDSS application, it was easy to use, and it did what it was meant to do. The accuracy of the app, as measured by percent of therapeutic target trough concentrations, was not different between the iOS CDSS application and the paper-based dosing nomogram (44.3%, paper nomogram, vs. 43.1%, iOS vancomycin CDSS application, p = 0.82;) as shown in Table 5.
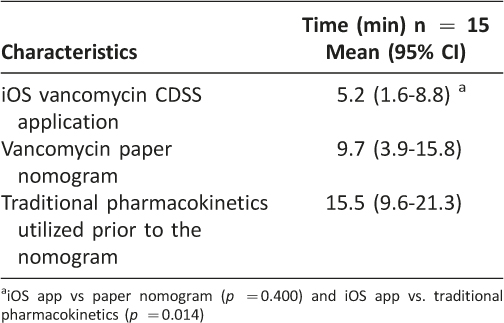
Table 3: Average Time Spent on Dosing Consults
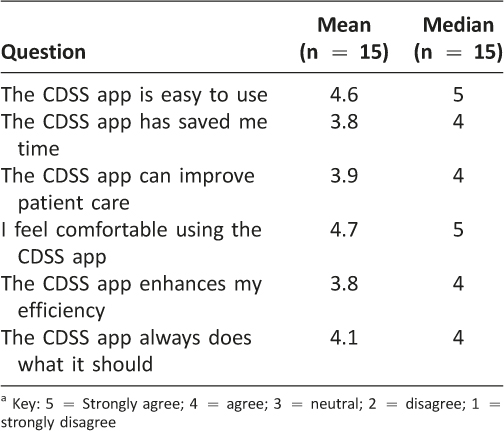
Table 4: Pharmacists’ Survey Results
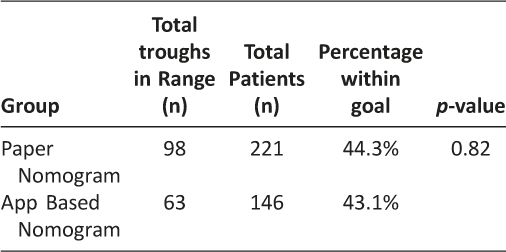
Table 5: Comparison of Dosing Methods to Predict Target Troughs
Discussion
Due to vancomycin resistance, updates in the vancomycin trough concentrations have been recommended. This increase in target trough concentrations has caused a need for the development of new vancomycin dosing nomograms. A novel vancomycin dosing nomogram was previously shown to meet these new standards, as well as, be significantly more accurate when compared to traditional pharmacokinetic calculations at our institution. These results led to the vancomycin dosing nomogram being adopted as the standard dosing protocol for all eligible patients at our institution.
Technology continues to have an enormous, positive impact in health-care. From using medication-counting machines to bar code technology, the use of technology has saved healthcare institutions significant time and money. With the evolution of iOS device technology, iOS device applications have started to play an integral role in healthcare. They are used in a variety of applications from obesity management to obtaining blood pressure estimates.14,15 Because of the significant role technology, and specifically iOS device applications play in health care, we decided to convert the vancomycin dosing nomogram into an iOS device application.
The present study was designed to prospectively evaluate the time and cost-savings realized by using the iOS vancomycin CDSS application. Our findings showed significant time and costs savings when using the iOS vancomycin CDSS application versus the traditional pharmacokinetic calculations method. The iOS vancomycin CDSS application was also found to be as accurate as the paper nomogram, pharmacists thought this tool was easy to use, and they felt comfortable using this tool. The paper version of the nomogram has already been shown to be superior to traditional pharmacokinetic calculations in a previous study.13 This equivalency in accuracy was not surprising due to the fact that this tool automated the calculations required by the paper nomogram, there are minimal input parameters needed to obtain the results, and the tool was developed to produce the exact results of the paper nomogram.
Based on the survey, there were several suggestions for improving the iOS vancomycin CDSS application. First, this tool displays the calculated dosing weight even if the patient is not 30% over his or her ideal body weight. Even though this parameter is not used in any of the calculations unless the patient is found to be 30% or more over his or her ideal body weight, the fact that it was always displayed caused several pharmacists to be concerned that this parameter was being used unnecessarily. Second, several pharmacists suggested that other pharmacokinetic parameters, such as half-life and the patient’s estimated elimination rate constant, be displayed on the results screen. Third, it was suggested that the institution specific infusion time be given instead of a generalized infusion time based on the dose of vancomycin administered. This feedback will be used to update the iOS vancomycin CDSS application.
This study had several limitations. First, the pharmacist survey used a convenience sample. Therefore the sample size was relatively small and may not be representative of all pharmacists’ impressions at our institution. Second, some pharmacists may have misinterpreted what was being asked regarding the amount of time it took to complete a vancomycin consult using the various dosing methods. The intention of the question was to evaluate the time it took to perform the calculations and determine a dose and the dosing interval, and not the time it took to fill out the consult form, decide what target trough needed to be achieved, decide if they were going to use the recommended dose, and complete the documentation of the consult. This confusion could have lead to lower cost-savings realized between the different dosing methods. Third, there was some reluctance by a few of the pharmacists to use this new tool in their normal workflow, which could have led to a reduction in the number of completed consults. We tried to mitigate this limitation by providing in-service training, user’s guides, and six iPad Minis with the application preloaded on each device. Fourth, there could have been a recall bias among pharmacists while filling out certain aspects of the surveys as it required them to think back to using the paper-based nomogram and traditional pharmacokinetic calculations. The paper-based nomogram had been in place as the standard for dosing vancomycin approximately 18 months prior to the start of this study. Fifth, the timing of trough concentrations and administration times of vancomycin were not always correct. Doses may be administered within one hour of the scheduled time at our institution, but it is not unusual for doses to be administered outside of this cut-off time. Finally, the value of $0.8814/min reflects a typical 40 hours per week and 52 weeks per year which underestimates the true cost per minute to the institution considering holidays, vacation, sick leave, personal days, health insurance subsidies, retirement account contributions, etc which typically equate to a fringe benefit rate of approximately 30% over base salary.
Overall, the iOS vancomycin CDSS application proved to be very easy to use and a cost effective clinical decision support software tool. Utilizing this software could allow pharmacists to reduce their vancomycin pharmacokinetic consult times allowing them to focus on other clinical duties. Future studies could focus on the cost savings this tool could provide if it completed the traditional pharmacokinetic dosing calculations for patients that could not be dosed using the current nomogram (e.g. body weight greater than 120 kg). Finally, the savings realized in this study were based on using the CDSS application with only a single model drug. Future studies could examine the time and cost savings of using CDSS applications with additional medications.
Conclusion
The use of this iOS vancomycin CDSS application has shown to be an accurate, time saving, and cost effective method for personalized dosage regimen determination in patients receiving vancomycin therapy. The tool allowed pharmacokinetic consults to be completed more efficiently and therefore has been adopted as the new standard vancomycin dosing method at our institution. The maximum time savings of 10.3 minutes per consult and cost savings of $13,617 per year was realized at our institution for just this single medication. The iOS vancomycin CDSS application will be updated based on feedback and additional drugs will be considered for incorporation into the CDSS application to expand its usefulness and cost savings.
Acknowledgements
The pharmacists of Winchester Medical Center’s Pharmacy Department, Lisa Hammond, Pharm.D., BCPS, Thomas Taylor, and Pam Lipscomb
References
1. Vancomycin. Clinical Pharmacology 2000, Gold Standard Multimedia Inc. (Accessed 2014 January 9).
2. Geraci J. Vancomycin. Mayo Clin Proc 1977;52:631–4.
3. Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Craig W, Billeter M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the american society of health-system pharmacists, the infectious disease society of america, and the society of infectious diseases pharmacists. Am J Health-Syst Pharm. 2009;66:82–98. ![]()
4. Liu C, Chanmers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents and Chemother. 2003;47:3040–5. ![]()
5. Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents and Chemother. 1984;25:433–7. ![]()
6. Moellering RC, Krogstad DJ, Greenblatt DJ. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med. 1981;94:343–6. ![]()
7. Rotschafer JC, Crossley K, Zaske DE, Mead K, Sawchuck RJ, Solem LD. Pharmacokinetics of vancomycin: observation in 28 patients and dosage recommendations. Antimicrob Agents and Chemother. 1982;22:391–4. ![]()
8. Lake KD, Peterson CD. A simplified doing method for initiating vancomycin therapy. Pharmacotherapy. 1985;5:340–4.
9. Nielsen HE, Hansen HE, Korsager B, Skov PE. Renal excretion of vancomycin in kidney disease. Acta Medica Scandinavica. 1975;197:261–4. ![]()
10. Kullar R, Leonard SN, Davis SL, Delgado S JR., Poque JM, Falcione B, et al. Validation of the effectiveness of a vancomycin nomogram in achieving target trough concentrations of 15-20 mg/L suggested by the vancomycin consensus guidelines. Pharmacotherapy. 2011;31:441–8. ![]()
11. Devabhakthumi S, Gonzales JP, Tata AL. Evaluation of vancomycin dosing and monitoring in adult medicine patients. Hospital Pharmacy. 2012;47:451–9. ![]()
12. Thalakada R, Legal M, Lau TTY, Luey T, Batterink J, Ensom MHH, Development and validation of a novel vancomycin dosing nomogram for achieving high-target trough levels at 2 Canadian teaching hospitals. Can J Hosp Pharm. 2012;65:180–7. ![]()
13. Wesner AR, Brackbill ML, Coyle LL, Kidd R. Prospective trial of a novel nomogram to achieve updated vancomycin trough concentrations. Interdiscip Perspect Infect Dis. 2013 Sep; Article ID 839456:1–8. ![]()
14. Anton SD, LeBlanc E, Allen HR, Karabetian C, Sacks F, Bray G, et al. Use of computerized tracking system on monitor and provide feedback on dietary goals for calorie-restricted diets: the pounds lost study. J Diabetes Sci Technol. 2012;6:1216–25. ![]()
15. Chandrasekaran V, Dantu R, Jonada S, Thiyagaraja S, Subbu KP. Cuffless differential blood pressure estimation using iOS devices. IEEE Trans Biomed Eng. 2013;60:1080–9. ![]()

