Development and Feasibility of a Tablet-based Self-monitoring and Management System in Pregnant Women
Development and Feasibility of a Tablet-based Self-monitoring and Management System in Pregnant Women
Hiroshi Kobayashi, MD, PhD1, Yuno Osanai2, Toshiyuki Sado, MD, PhD1, Katsuhiko Naruse, MD, PhD1, Michiyuki Horiguchi3
1Department of Obstetrics and Gynecology, Nara Medical University, Nara, Japan; 2ShinIng. Inc., Otaru, Japan; 3Rastec, Co. Ltd., Nara, Japan
Corresponding Author: hirokoba@naramed-u.ac.jp
Journal MTM 6:1:19–25, 2017
Objectives: Many health telemedicine applications (apps) have been released in the clinical-care setting. There is limited information regarding the utility of ICT-based maternity data collection and a comprehensive home-based model of care for high risk pregnant women. The aim of our study was to assess the feasibility of a tablet-based real-time bidirectional telecommunication of self-reported maternal condition in normal and high risk pregnant women.
Methods: The study included eighteen pregnant women (13 normal pregnant women [the Cohort 1 study] and 5 high risk pregnant women [the Cohort 2 study]) who expressed their interest in participating in the study. Participants were supplied with a tablet computer installed with the “MaternityCare” service program. Each user recorded physical information (body weight and blood pressure) and answered the multiple questions on obstetrical symptoms daily for 30 days.
Results: The median value of individual compliance with practice (individual patient-level reporting rates) was 76.7% in the Cohort 1 study and 100% in the Cohort 2 study. Self-reporting data were transmitted on 65.8% for the Cohort 1 study, and 218.9% for the Cohort 2 study of subject days (subject day =1 subject × 1 day). 66.7% (10/15) of participants affirmed applications’ ease of use, and 60% (9/15) desired to implement the app into practice after the study.
Discussion: In conclusion, high risk pregnant women had a positive attitude about home-based self-monitoring and expressed a strong desire to receive this app. A dynamic, real-time, bidirectional and interactive mobile maternity health system with a tablet app may support information sharing, quick consultation and initiation of the prophylaxis and treatment at the patient, pre-hospital healthcare provider and physician levels.
Introduction
Online social networking facilitates communication between users. The use of smart technology, including a tablet or smartphone, in daily life has become ubiquitous and enables not only telephone calls, but also allows constant access to communication and information.1,2,3 Information and communication technology (ICT)-based health technologies, electronic health (e-health) or mobile health (m-health), have been used for disease self-management, patient education, professional education, or patient monitoring.3,4 With the advances in ICT, many health telemedicine applications (apps) have been released in the clinical-care setting in recent years.4 The speed of development in internet technology for telemedicine in patients with chronic diseases has been rapidly accelerating. However, to the best of our knowledge, through literature reviews, there is limited information regarding the utility of ICT-based maternity data collection and a comprehensive home-based model of care for high risk pregnant women who want to identify obstetric problems earlier and increasing demand for their need and care, implemented through an e-healthcare software.5,6 We have developed a new model for maternity e-healthcare devices using a tablet-based application that provides a bidirectional communication through e-mails and mobile text messages between pregnant women and trained coordinators/obstetricians and offers medical advice. The objectives of this study were 1) to determine the usability, feasibility, preliminary efficacy and safety of a tablet-based real-time bidirectional telecommunication of self-reported maternal condition such as body weight, blood pressure and daily symptom questionnaire for 30 days in normal and high risk pregnant women, and 2) to evaluate the effect of a bidirectional and multi-group telemedicine system on satisfaction in these pregnant women.
Methods
Study population
A single-center, prospective, pilot study evaluated overall satisfaction, ease of use, flexibility, accuracy, stimulation and information contents of the telemonitoring system in order to determine the impact of the maternal data on self-care and clinical management. A Cohort 1 study initially targeted normal pregnant women who visited an obstetric outpatient clinic of Nara Medical University Hospital, Kashihara, Japan, from January 2014 to June 2014. Only those pregnant women who met the following maternal inclusion criteria were selected: singleton pregnant women, 20 years of age or older, their first prenatal visit in our hospital before 12 weeks 0 days of gestation, ability to speak and read in Japanese and ambulatory patients diagnosed without any obstetric complications. Women were excluded from the Cohort 1 study if they have any of the following risk factors or conditions: pregnancy-induced hypertension, history of gestational diabetes mellitus (GDM) or pre-existing DM, body mass index (BMI) >30 kg/m2, or any medications. Participants not willing to have a series of prenatal care visits, with an unknown number of prenatal visits, or no prenatal care were excluded from the study. Furthermore, in a prospective Cohort 2 study, recently discharged high risk pregnant women hospitalized for pregnancy-induced hypertension were invited to participate in the feasibility study between July 2014 and December 2014. The inclusion criteria were the same for Cohorts 1 and 2. The exclusion criteria of Cohort 2: history of GDM or pre-existing DM, BMI >30 kg/m2, and participants not willing to have a series of prenatal care visits, with an unknown number of prenatal visits, or no prenatal care. Owing to small sample sizes, descriptive statistics using the sample size estimation method were not presented.
Participants were invited to receive information from the study coordinator regarding participation in the feasibility study and recruited during face-to-face visits. All eligible patients who agreed to participate in the study were interviewed by trained study coordinators. Two certified midwives were trained as the study coordinator. Eighteen pregnant women (the Cohort 1 study, n=13; and the Cohort 2 study, n=5) were initially eligible for participation. Ethical approval was obtained from the Ethics Committee at Nara Medical University. Informed consent was required for participation in this study.
Daily Collection via the App
Participants were provided with a medical app for tablet computers (SHARP Tablet-type, RW-T110, http://www.sharp.co.jp/business/tablet/products/rwt110.html) installed with a “MaternityCare” app for the personal use. They received the medical app free of charge and no longer received any reimbursement of expenses for extra data usage caused by the app utilization. After launching and downloading the app, the authentication of a legal user could be exercised by username ID and password. A 15-minute negotiated interview with each participant on enrollment in the Cohort studies included the following items: instructions on how to log in to the app; entering baseline demographics such as socio-demographic variables (name, age, marital status, and husband’s occupation), obstetrical situation (parity, history of abortion, type of previous delivery) and physical information (height, weight and blood pressure) into the app. Participants were presented with all of the functional components of the app in a tab-accessible format at any time from the day following provision of written consent and could start reporting their maternal condition and vital data via the app (Figure 1). Figure 1A presents a screenshot of the web-based and mobile app on a tablet. The individuals recorded body weight and blood pressure (Figure 1B). The self-measurement of blood pressure was obtained by each individual as a home blood pressure monitoring. Once-daily measurements are recommended, usually in the morning. When blood pressure measurements are at least >90 mm Hg diastolic or >140 mm Hg systolic, the app recommends repeating measurements. Data were confirmed after repeating measurement to prevent false readings. If repeating measurements were blood pressure >140/90 mmHg, the app sends an automatic email to a study coordinator. Furthermore, each user was asked to complete a tablet-based self-report screening questionnaire about the maternal condition in order to determine the feasibility and acceptance of the implementation of novel technology. The maternity information was assessed using established questionnaires in Japanese on current status that included items on vaginal discharge, vaginal bleeding, emesis, vomiting, abdominal pain, uterine contraction, fetal movement, edema and questions for measuring self-care and quality of life (Figure 1C, left column). Symptoms were entered daily using on-screen radio button selection of information related to symptoms. The questionnaires in the practice and attitude sections consist of 6 categories (overall satisfaction, ease of use, flexibility, accuracy, stimulation and information contents) containing 12 questions that use a 4-5-point Likert scale based on a ‘yes or no’ response or ‘mild, moderate or severe’ to a question. The daily physical measurements took about 5 minutes each morning or evening. After the questions were answered without missing values and outlier data, the user could send validated self-reported physical information and obstetrical symptoms daily, and then received electronic assessment, advice and alerts via an automated search algorithm by our clinical decision support system. Data were captured wirelessly and stored in a secure database. The app provides participants and the study coordinator the ability to view multiple summary graphs of the maternal condition data (Figure 1C, right column). The technological (feedback on automated electronic assessment and health status via the app) and non-technological interventions (via the coordinators/obstetricians) are essential to ensure fail-safe electronic communication and follow-up of critical alert notifications and to reduce missed test results of tracking processes. Scores of ≥ moderate or severe on either questionnaire generated an automated electronic referral to the study coordinator in real time.
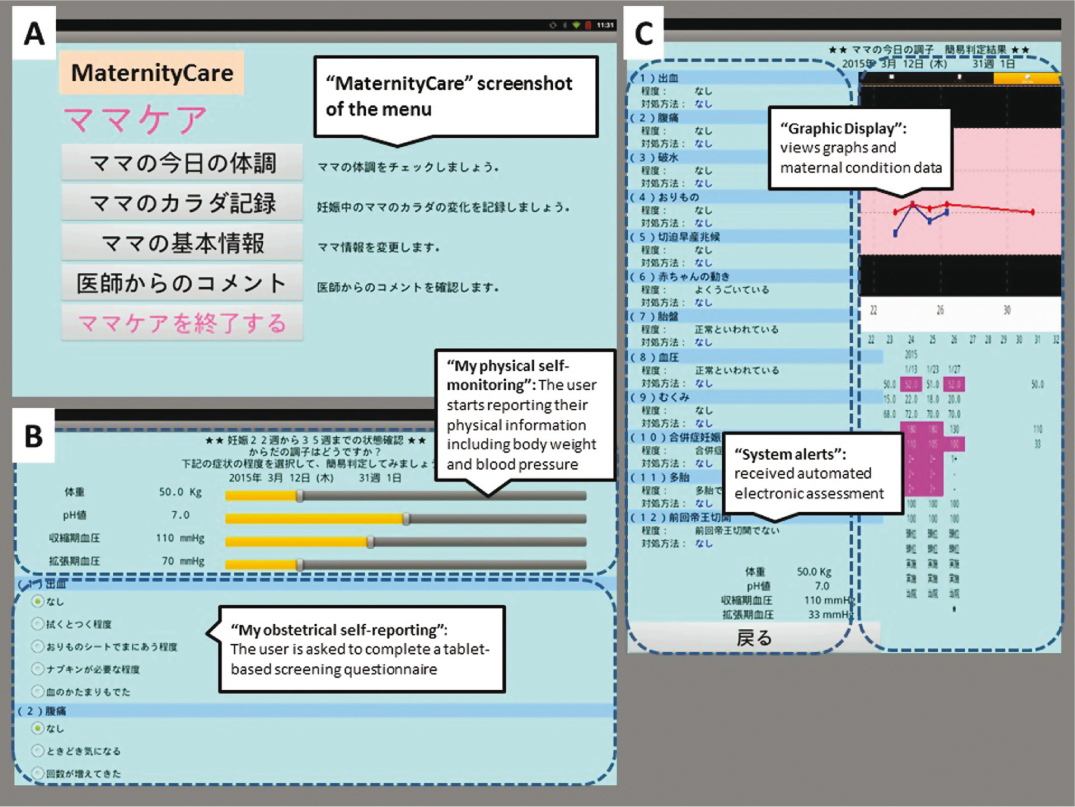
Figure 1: “MaternityCare” screenshot of the menu, the graphic display, and the self-reporting of maternity information data. This figure presents the features associated with the following: A, “Screenshot of the menu” (the welcome screen of the web-based and mobile “MaternityCare” app); B, upper part, “My physical self-monitoring” platform (entering body weight and blood pressure data); B, lower part, “My obstetrical self-reporting” (daily symptom questionnaire); and C, left part, “System alerts” (feedback on automated electronic assessment and health status); and C, right part, “Graphic Display” (the visual data in real time)
Participants were also advised to report their symptoms through the tablet computer if they suffer from specific or non-specific ill-health during that day. They can also send text messages and numerical obstetrical data to the study coordinator using the electronic keypad. If the study coordinator would likely seek consultation from an obstetrician, text messages from participants will be emailed from the study coordinator to the obstetrician’s mobile phone. If the obstetrician determined contacting the patient was warranted, a final message or alert was sent from the obstetrician to the mobile phones of the patient via the study coordinator. The participants, the study coordinator and the obstetricians are able to view their symptoms and the physiological data in text messages and graphic formats. The tablet app-based platform used the flexibility of real-time bidirectional flow communication. The alerts from the study coordinator to the patient ranged from low priority health conditions in clinical practice; to retake the measurements of physical information if the patient feels ill-health, to high priority alarms; to be a potentially important clinical event, to go to the emergency department, or to call their home doctors for an unscheduled visit.
System design and development have been guided by an expert steering committee. The “MaternityCare” app was developed by Rastec Co. Ltd., Nara, Japan. This app was based on two preliminary frameworks: 1) “My physical self-monitoring”: a calendar feature for recording body weight and blood pressure, and 2) “My obstetrical self-reporting”: a tablet-based screening questionnaire for bidirectional telecommunication between participants and study coordinator. The study coordinator can use the visual data in real time to provide better health care services to target user.
Data analysis
Box-and-whisker plot analysis was performed for data on reporting rates to determine quartiles and interquartile ranges. The box plot contains a central rectangle with lines that extend from both ends. It provides the information about the smallest value, first quartile, median, third quartile, and the largest value.
Results
Total volume of data transfers between server and subject
Figure 2 shows participant flow diagram over inclusion/exclusion process. Thirteen normal pregnant women expressed their interest in participating in the Cohort 1 study. After informed consent, one woman refused to participate in the study due to lack of interest. Thus, 12 cases (median age 31 years; range 24–38 years) were selected to enter the Cohort 1 study. Of the 12 eligible participants, two did not start to use the app or did not report self-reporting symptoms at all, although they did not feel technical problems in using their tablet (Table 1, cases 7 and 12). Among the 5 high risk pregnant women initially enrolled in the Cohort 2 study, two were excluded for the following reasons: incompatibility between the app and a tablet (n=1) and lack of interest in the study (n=1). A total of 3 patients (median age 32 years; range 26–39 years) were evaluated in the Cohort 2 study. The cumulative probability of dropout showed 23.5% (4/17) of all participants during the study. Finally, the survey was completed by 13 (13/17, 76.5%) individuals.
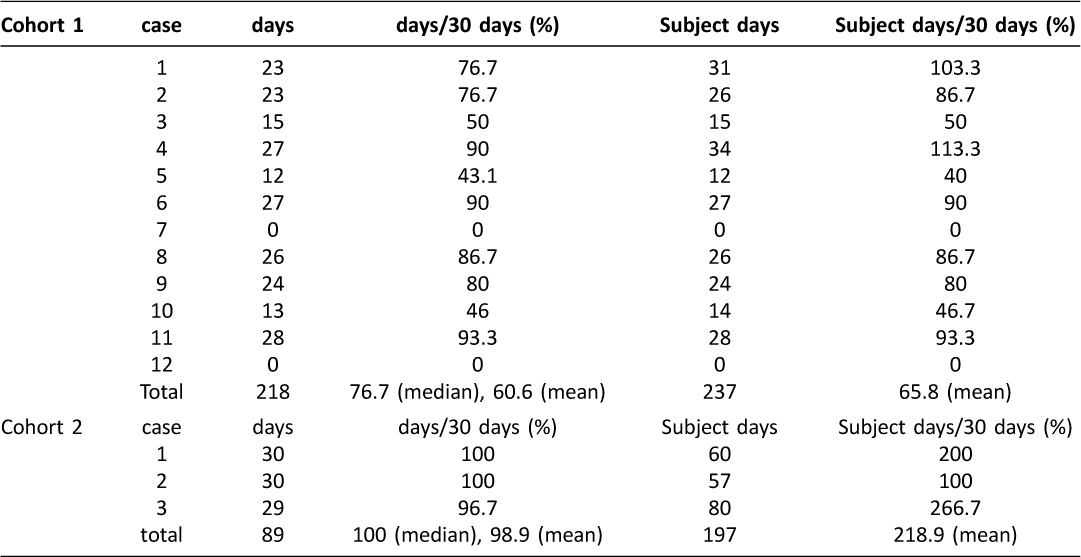
Table 1: The value of self-reporting data in the Cohort 1 and Cohort 2 study
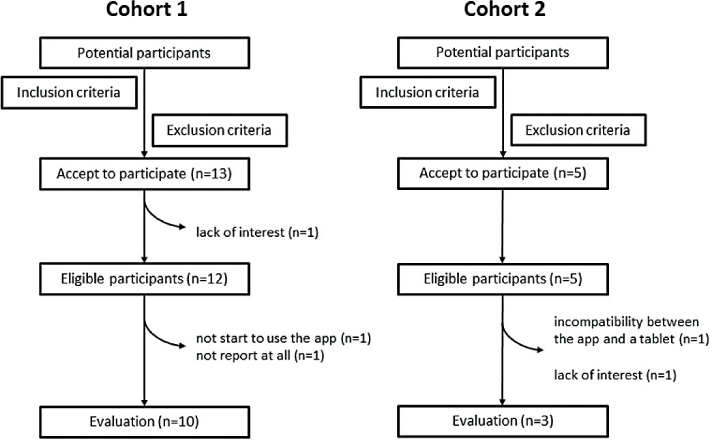
Figure 2: Participant flow chart over inclusion/exclusion process
The median value of individual participant-level reporting rates in the Cohort 1 and Cohort 2 study was 76.7% (range 0–93.3%) and 100% (range 96.7–100%), respectively (Table 1 and Figure 3). As shown in Table 1 and Figure 4, 237 and 197 self-reporting data were collected via their tablets for 30 days, respectively (overall self-reporting rate = 65.8%, 237/12×30 for the Cohort 1 study and overall self-reporting rate = 218.9%, 197/3×30 in the Cohort 2 study). During the current study, none had criterion for uncontrolled symptoms or adverse obstetric outcome that require additional intervention, including abnormal uterine hemorrhage, preterm premature rupture of membrane, preterm birth, pregnancy-induced hypertension, preeclampsia, HELLP syndrome, gestational diabetes mellitus and uterine abruption.

Figure 3: Box and whisker plots representing median levels and the interquartile range (box) of individual participant-level reporting rates for each studied group
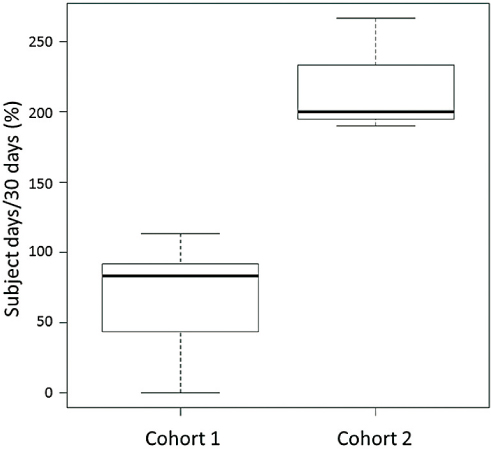
Figure 4: Box and whisker plots representing median levels and the interquartile range (box) of overall self-reporting rates for each studied group
Satisfaction questionnaire for the application: primary reasons for missing self-reporting data
The results of the patient’s perceptions of this system are shown in Table 2. They were evaluated for satisfaction questionnaire for the application from 6 categories. Despite being at different cohorts of pregnancy (normal and high risk), the results and discussion were centered around both groups together owing to small sample size. Of the 15 respondents, 10 users expressed overall satisfaction in fulfilling the required task and expectations. More than half (66.7%, 10/15) affirmed applications’ ease of use, and 9 women desired to implement the “MaternityCare” app into practice after the study. The remaining 5 patients expressed dissatisfaction accompanied with problems, practical drawbacks and complaints. The most common reason for missing self-reporting, which accounted for 60.0% (3/5) of all responses, was “lack of experience in the use of technology”. Other reasons included “inconsistent usage” and “Password login errors, Data transfer errors, Network errors”. No missing data were due to “I was too busy”.

Table 2: Overall responses of participants to the six items
Discussion
We designed and developed a mobile telemedicine application as health interventions for self-monitoring, reporting and management for normal and high risk pregnant women. This system contains two algorithms: one is to instigate warnings to the mother if there are premonitory signs or symptoms of a potential adverse event; and the other is that the data is independently reviewed by trained study coordinators and/or obstetricians. The current bidirectional telemedicine system can transmit physical information and obstetric complications/symptoms to the coordinators, and deliver real-time information with risk-reduction advice back to the user from the study coordinators and/or obstetricians. We aimed to assess the feasibility of a prototype “MaternityCare” tablet app in normal and high risk pregnant women. 76.5% (13/17) of the participants used the tablet app. Subjects accepted the tablet app were satisfied with its functionality and ease of use and 60% of participants desired to continue using it beyond the pilot. Women who are at high risk of pregnancy complications were more likely to use the tablet app compared to normal pregnant women. Preliminary results of this study indicated the general acceptance by pregnant women in using this system (Figures 3 and 4). However, the findings showed that the research model had poor explanatory power.
Most telemedicine and e-health self-care apps have focused on fields such as chronic conditions.7 There are many studies on intensive and extensive bidirectional telemedicine research into the obstetrical area.8,9 Despite the advance of e-health technologies through electronic devices, there are relatively few studies that have evaluated the management of high risk pregnant women.5,6 There may be an increased demand for bidirectional e-health self-care telemedicine using a smartphone app in high risk pregnant women.
The results of the present study should be interpreted with caution. First, these results have limited generalizability because of the enrollment only of Japanese pregnant women at a single tertiary center. Second, the apparent limitation of the study is a small number of patients. We have to be careful in making the conclusion that the use of the app is “feasible” in the high-risk pregnancy group as only 3 patients were included in this group. Finally, this study is limited by its non-randomized nature. Therefore, we cannot exclude the possibility of bias in our evaluation because of a lack of a control group.
In conclusion, this telemedicine provided the e-healthcare system of care for normal and high risk pregnant women to send automated and manual feedback through trained study coordinators and/or obstetricians. A dynamic, real-time, bidirectional and interactive mobile e-health system with a tablet app may support physical and obstetric information sharing, knowledge translation, quick consultation and initiation of the prophylaxis in the high risk pregnant women. Further research should focus on high risk pregnant women for mobile e-health care: how to facilitate the promotion of self-management and how to sustain compliance for a longer time.
Conclusions
We assessed the feasibility of a tablet-based real-time bidirectional telecommunication of self-reported maternity data in normal and high risk pregnant women. This study found that high risk pregnant women had a positive attitude about home-based self-monitoring and expressed a strong desire to receive this app. A dynamic, real-time, bidirectional and interactive mobile maternity health system with a tablet app may support information sharing, quick consultation and initiation of the prophylaxis and treatment at the patient, prehospital healthcare provider and physician levels.
Funding
The financial support was used to cover the expenses incurred by utilization of the app. This study was funded by Ministry of Economy, Trade, and Industry, Japan as a part of “Program to support prototype development by manufacturing SMEs and micro-enterprises”.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Patrick K, Griswold WG, Raab F, Intille SS. Health and the mobile phone. Am J Prev Med 2008;35:177–81. ![]()
2. Mosa AS, Yoo I, Sheets L. A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak 2012;12:67. ![]()
3. Chhablani J, Kaja S, Shah VA. Smartphones in ophthalmology. Indian J Ophthalmol 2012;60:127–31. ![]()
4. Okazaki S, Castañeda JA, Sanz S. Clinicians’ assessment of mobile monitoring: a comparative study in Japan and Spain. Med 2 0 2013;2:e11. ![]()
5. Martínez-Fernández A, Lobos-Medina I, Augusto Díaz-Molina C, Faraón Chen-Cruz M, Prieto-Egido I. TulaSalud: an m-health system for maternal and infant mortality reduction in Guatemala. J Telemed Telecare 2015;21:283–91. ![]()
6. Carral F, Ayala MD, Fernández JJ, González C, Piñero A, García G, Cañavate C, Jiménez AI, García C. Web-Based Telemedicine System Is Useful for Monitoring Glucose Control in Pregnant Women with Diabetes. Diabetes Technol Ther 2015;17:349–54. ![]()
7. Norman GJ, Zabinski MF, Adams MA, Rosenberg DE, Yaroch AL, Atienza AA. A review of eHealth interventions for physical activity and dietary behavior change. Am J Prev Med 2007;33:336–45. ![]()
8. Lund S, Hemed M, Nielsen BB, Said A, Said K, Makungu MH, Rasch V. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: a cluster-randomised controlled trial. BJOG 2012;119:1256–64. ![]()
9. Ngabo F, Nguimfack J, Nwaigwe F, Mugeni C, Muhoza D, Wilson DR, Kalach J, Gakuba R, Karema C, Binagwaho A. Designing and Implementing an Innovative SMS-based alert system (RapidSMS-MCH) to monitor pregnancy and reduce maternal and child deaths in Rwanda. Pan Afr Med J 2012;13:31.

