Remote Biochemical Verification of Smoking Abstinence via Mobile-Phone Video Call
Remote Biochemical Verification of Smoking Abstinence via Mobile-Phone Video Call
Sun S. Kim1, Kunsook Bernstein2, Olivia Shim3, Hua Fang4, Sherry McKee5, Douglas Ziedonis6
1University of Massachusetts Boston, College of Nursing and Health Sciences; 2Hunter College City University of New York, School of Nursing; 3Felician College, Department of Education; 4University of Massachusetts Dartmouth, Department of Computer and Information Science; University of Massachusetts Medical School, Department of Quantitative Health Sciences; 5Yale School of Medicine, Department of Psychiatry; 6University of California San Diego, Department of Psychiatry
Corresponding Author: sun.kim@umb.edu
Journal MTM 7:1:1–8, 2018
Introduction: This study tested the feasibility of a home-based salivary cotinine test for the remote verification of smoking abstinence via a mobile-phone video call.
Methods: Korean American women were recruited into a pilot smoking cessation study by advertising the study in online Korean American women’s communities and offline Korean ethnic newspapers. Smoking abstinence was based on a combination of self-report and salivary cotinine test at post-quit 3-month follow-up. Those who self-reported smoking abstinence were invited to conduct a home-based salivary cotinine test using a NicAlertTM test strip. Research staff monitored the whole process of the test via a mobile-phone video call and read the result when it was ready.
Results: At 3-month follow-up, 20 women (20/49, 40.8%) reported smoking abstinence. Of the 20, 16 (80.0%) performed the home-based salivary cotinine test; three refused the test; and one was excluded due to the use of nicotine patches. All but one yielded a level of 0 (cotinine concentration ≤ 10ng/ml) on the test strip, which indicates abstinence. The women who yielded a higher level than 0 or did not perform the test were all treated as smokers. Thus, the rate of biochemically verified smoking abstinence was 30.6% (15/49).
Conclusion: There was a substantial difference (> 10.0%) between self-report and biochemical verification although the difference was not statistically significant. The verification seems to be crucial for the accurate assessment of smoking abstinence in Korean American women who tend to underreport use.
Keywords: mobile-phone technology, smoking, nicotine addiction, salivary cotinine test, and biochemical verification.
Introduction
The Society for Research on Nicotine and Tobacco (SRNT) Subcommittee on Biochemical Verification recommended that biochemical testing is not necessary for large population-based or low-intensity intervention studies.1 The recommendation was made more than a decade ago. As smoking becomes increasingly unacceptable, many smokers are now more likely than ever to underreport the use.2–4 In one smoking cessation study, the sensitivity of self-reported abstinence was low at 29%, whereas the specificity was 100% when compared with the results of a urinary cotinine test, using a cutoff point of 50ng/ml.5 Another study also reported a substantial difference in abstinence rates between self-report and biochemical measure even though it provided a low-intensity smoking cessation treatment such as brief face-to-face plus telephone counseling.6
The veracity of self-reported abstinence has been established by large population-based surveys with the general population. However, some subgroups of smokers, such as pregnant women, youth, and racial and ethnic minorities, are likely to underreport use. For example, nondisclosure rates were much higher among pregnant active smokers (22.9%) than non-pregnant active smokers (9.2%).7 In the same study, non-pregnant Mexican and African American smokers (23.0%) were more likely to conceal their smoking than were non-pregnant White smokers (7.9%). Ample evidence also exists that Asian Americans, particularly women, are likely to underreport.8–11 A large population-based survey study in Korea found that more than half of the women (59%) who were determined to be smokers based on urinary cotinine test self-reported as non-smokers.12
When the SRNT Subcommittee on Biochemical Verification published their recommendations,1 the optimal data collection method of a bodily fluid sample was through the mail and hence, the sample could be easily tainted if someone wanted to underreport use. Furthermore, biochemical tests then could be done only in a laboratory setting, and the cost was highly expensive. However, the availability of salivary cotinine test kits and the advent of video call web applications have enabled researchers to conduct a home-based biochemical test with a modest cost ($12-15 per test).13 The source of a bodily fluid sample can be easily validated by having a person spit into a collection tube during a video call. Cotinine is the first-stage primary metabolite of nicotine and has a long half-life of approximately 15-19 hours.14 Therefore, it has been used as a reliable biomarker of nicotine exposure.
Dallery and colleagues15,16 conducted a study of voucher-based contingency reinforcement by remotely validating smoking abstinence with breath carbon monoxide (CO) tests. There are several types of commercially available CO meters that measure breath CO in parts per million (ppm) based on the conversion of CO to a catalytically active electrode. CO meters are highly expensive ($800-1200 per piece) and may not be affordable for most researchers and clinicians. More recently, an attempt was made to develop a mobile-phone-based breath CO meter, and its preliminary measures showed significant correlations with those from a commercially available CO meter.17 The mobile-phone-based CO meter is still in the early stage of development and not currently available for use.
Unlike the overall decline in smoking prevalence observed across other racial and ethnic groups, the rate for Korean American women has been on the rise.18,19 This observation is alarming given that the group is likely to underreport smoking because of the strong cultural taboo against the use by women in Korea and within Korean American communities.10 For example, more than half of the Korean American women who participated in a smoking cessation study reported that they had been keeping their smoking secret from others.20 The rate of smoking among Korean American women could be much higher than what has been reported. What might have contributed to the increase is beyond the scope of this study (for interested readers, please read the article by Kim and others10).
There is an urgent need for a smoking cessation intervention that is effective for Korean American women and its effectiveness should be evaluated using a biochemically verified abstinence rate. The present study was conducted to assess the feasibility of a home-based salivary cotinine test via a mobile-phone video call. It was assessed by estimating the rate of participation among those who were invited for the test and also the rate of results that were readable among the tests conducted. The test enables a researcher to verify self-reported smoking abstinence of participants who are remotely participating in a smoking cessation study. Breath CO test was found to be not sensitive enough to detect underreports of smoking in Korean Americans.20
Methods
Participants
The present study used data from a 2-arm randomized controlled trial testing the feasibility and acceptability of a videoconferencing smoking cessation intervention for Korean American women.21 The study compared the relative effectiveness of a videoconferencing intervention (video arm) with a telephone voice call intervention (voice arm). Selection criteria for participants are described in Table 1. Participants were recruited by advertising the study in various Korean online communities and offline Korean newspapers in the United States. A total of 49 Korean American women were recruited into the study and approximately 44.9% of them were from the west coast, 36.7% from the east coast, and the remaining were from southern or central states. The study was approved by the Institutional Review Board of the University of Massachusetts Boston. All participants provided a signed informed consent form via a regular postage mail or e-mail.
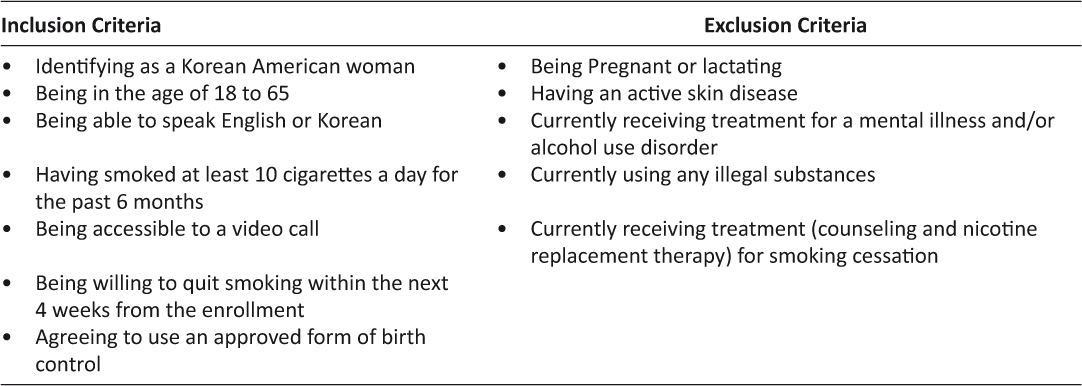
Table 1: Selection criteria for participants
Intervention Procedure
Participants were randomly assigned to either arm at a ratio of 1:1. First, numbers were randomly generated by a computer-based randomizer and then the generated numbers were sorted by the arm. Each number was transferred to a paper and a black dot was circled next to the number if it belonged to the video arm. The paper was then stored in a sealed envelope that had the same number written on the outside. When a new person completed an informed consent form and baseline questionnaires, the envelope was torn off, and the person was assigned to either arm based on whether or not the paper inside had a black dot. Both arms received the same cessation intervention—that is, a combination of 8 weekly individualized counseling sessions, each for 30 minutes, and 8 weeks of active nicotine patches. The only difference was the delivery mode of intervention: mobile-phone voice call versus mobile-phone video call. Quit day was set between the third and fourth counseling sessions. A detailed description of the procedure has been reported elsewhere.21
Data Collection
Baseline assessment was done via telephone interviews or self-administration by mail delivery. All measures had been translated and back-translated through a rigorous process of cross-cultural validation and pilot-tested with Korean Americans before the use. Findings pertaining to their psychometric functions have been reported elsewhere.7,22,23
Demographic data. They included age, marital status, years of education, family income, employment, and medical insurance coverage.
Acculturation. It was assessed using a brief version of the Suinn-Lew Asian Self-Identify Acculturation Scale.24 Instead of the full 21-item scale; a brief 5-item scale was used (e.g., one’s familiarity with spoken and written languages between English and Korean and preference for ethnic identity). These items had the highest item-to-total correlations among the 21 items.25 The score of each item ranges from 1, “Korean-culture oriented,” to 5, “American-culture oriented” and the average of the scores of the five items is the scale score. Cronbach’s alpha that is an indicator for the internal consistency of the measure was 0.74.
History of smoking. The following information was gathered: age at onset of regular smoking, any indoor-house and office smoking within the past 7 days, the presence of other smokers in the family, the average number of cigarettes smoked per day, and any 24-hour or longer abstinence as a quit attempt in the past year. In addition, participants were asked about whether they had kept their smoking secret from family, friends, or healthcare providers.
Nicotine dependence. The Fagerström Test for Nicotine Dependence (FTND) was used. The total score is the sum of the scores of six items and ranges from 0 to 10, and high scores indicate more dependence on nicotine.26 A Cronbach’s alpha of 0.32 was obtained in this study. A previous study testing the psychometric function of the measure reported that the measure was neither valid nor reliable for use with Korean American women.23
Autonomy over Tobacco Scale (ATS). This measure is a reliable and valid measure of nicotine addiction, assessing diminished autonomy over tobacco use.27 It consists of 12 question items assessed on a 4-point scale ranging from “not at all to “very well.” Its face validity and other psychometric functions have been validated for the general U.S. population.28 Because of the poor psychometric findings of the FTND for Korean women, the ATS was also used. To our knowledge, this measure had never been used with Korean Americans before the present study. It was translated and back translated with a rigor required for cross-cultural validation.21 A Cronbach’s alpha of 0.87 was obtained in this study.
At post-quit assessment, use or non-use of any tobacco products was assessed weekly during the first 4 weeks of quitting and then monthly until post-quit 3 months. The primary outcome was 7-day point prevalence abstinence meaning that an individual had not smoked a single puff for the past 7 days,29 which was assessed at each follow-up. The assessment was based on self-report at the first two follow-ups because of the use of nicotine patches and a combination of self-report and saliva cotinine test at the third follow-up. Participants were frequently (five times) informed throughout the study from recruitment to each follow-up assessment that they must perform a home-based salivary cotinine test for the verification of smoking abstinence at the 3-month follow-up, and research staff would assist them via a mobile-phone video call.
The NicAlertTM test costs $12-15 and is based on the principle of enzyme-linked immunosorbent assay; the test strip displays seven zones that represent a range of cotinine levels from 0 (cotinine concentration 0-10 ng/ml) to 6 (cotinine concentration ≥ 2000 ng/ml).13 Given that the standard salivary cotinine concentration cutoff for determining smoking status is 14 ng/ml, a cotinine level of 0 (cotinine concentration <10 ng/ml), 1 (cotinine concentration 10–30 ng/ml), and 2-6 (cotinine concentration > 30 ng/ml) indicate nonsmoking, secondhand smoke exposure or low-level smoking, and regular smoking, respectively.30 Compared with gas chromatography, the NicAlertTM had a specificity of 95% (95% CI [89%, 100%]), a sensitivity of 93% (95% CI [85%, 100%]), a positive predictive value of 95% (95% CI [89%, 100%]), and a negative predictive value of 93% (95% CI [86%, 100%]).30
The NicAlertTM test was standardized and monitored via a mobile-phone video call. Participants who self-reported abstinence for the past 7 days at the 3-month follow-up received one single NicAlertTM test kit within 2-3 days via express mail. When participants confirmed the arrival of the kit, research staff scheduled the test and assisted them in setting up a video call application if they had never used the application before. Participants were instructed not to open the sealed test strip until the scheduled test time. They were also instructed not to eat or drink anything for at least 30 minutes before the test.
Participants first tore off the sealed envelope to take out the test strip and lay on a dry flat surface with the numbered levels facing up. They spat into a collection tube through a small funnel until the tube was half full. The funnel was then discarded, and the tube was capped. Lastly, they held the tube upside down and squeezed until at least eight full drops of saliva dripped onto the white padded end of the test strip. A female research staff closely monitored the whole process via a mobile-phone video call. She was not an interventionist but was not blind to the treatment condition because she assisted some participants in the video arm to set up a video call application and transcribed audiotapes of therapy sessions for the assessment of intervention fidelity. The test result was read by the staff in about 20-30 minutes when the blue band on top of the test strip disappeared. While awaiting for the result, the staff administered follow-up research questionnaires over the phone. Participants took a photo of the test strip right after the video call and sent it to the staff member. The photo was then independently read by a third person, and the readings by the staff member and third person were compared for interrater reliability. Those who yielded a result that might not be in agreement with their self-report would be asked to repeat the test in a week.
Data Analysis
The parent study was conducted as a pilot study for the estimation of a preliminary effect size comparing a videoconferencing smoking cessation intervention with a telephone counseling smoking cessation intervention. For a pilot study, it was suggested that 24 subjects per arm generally yields near an accurate estimate of an effect size.31 All data analyses were performed using the Statistical Package for the Social Sciences (New York, USA) version 22.0. The feasibility of a home-based salivary cotinine test was the main objective of the present study, and hence, descriptive statistics of demographics and smoking-related variables were performed with the total sample without comparison between the video and voice arms. Interrater agreement was assessed using Cohen’s Kappa statistics. Participants who self-reported abstinence but refused to do the salivary cotinine test were all treated as smokers. Based on the intention-to-treat analysis, those who were missing at follow-ups were also treated as smokers.
Results
Forty-nine Korean American women participated in the study. Demographic characteristics and smoking behaviors are presented in Table 2. The mean age of the women was 45.1 years (SD = 12.1) and the mean age of smoking onset was 23.1 years (SD = 6.3). The average score of acculturation was 1.9 indicating that the majority of the participants were Korean-culture oriented. Almost three out of four women (71.4%) had not disclosed their smoker’s status to family and/or friends, and more than one-third of the women (36.7%) had not disclosed to healthcare providers.
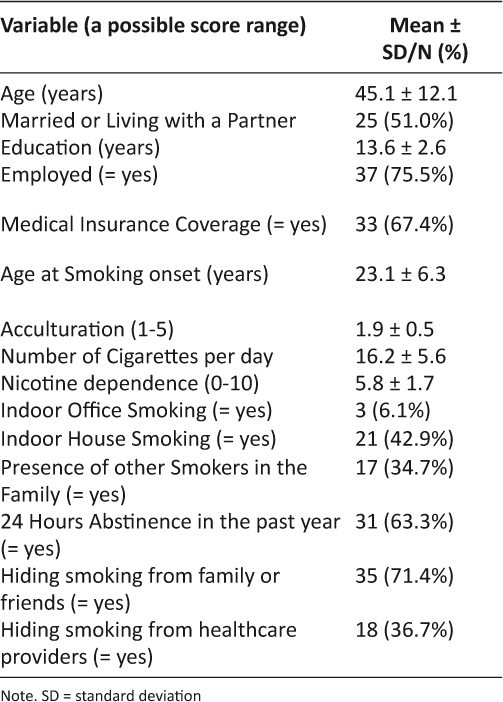
Table 2: Comparison of baseline characteristics (N = 49)
At 3-month follow-up, 20 (40.8%) reported abstinence for the past 7 days. Of these, one woman was excluded from the test due to the report that she was using nicotine patches at the time. Nineteen women received the NicAlertTM test kit, but three women (15.8%) did not conduct the home-based test. The test took approximately 25 to 30 minutes from opening a test kit to obtaining the result of the test. The 16 women who performed the test all yielded a result that was readable, and all but one yielded a level of 0 (≤ 10ng/ml, Figure 1) showing an agreement with their self-report. One woman who yielded level 2 was retested in 5 days, but the result was level 1. Thus, she was treated as smokers. There was no discrepancy (Kappa = 1.0) in readings of the results between the two raters. Those who were missing at the follow-up (n = 11) and who did not perform the test (n = 4) were all treated as smokers.
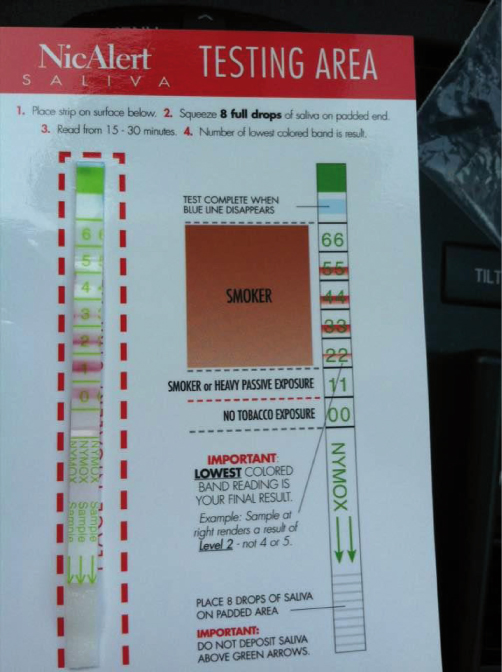
Figure 1: A picture of NicAlertTM test strip yielding a level of 0
Figure 2 presents the rates of 7-days point prevalence abstinence from post-quit 1 month to 3 months based on self-report. The rates were almost the same between the first (57.1%) and second month of quitting (55.1%), whereas the rate suddenly dropped to 40.8% in the third month when the salivary cotinine test was requested. The rate further declined to 32.7% when it was verified with the NicAlertTM test strip. There was no difference between the self-reported and cotinine-confirmed abstinence rates at post-quit 3 months.
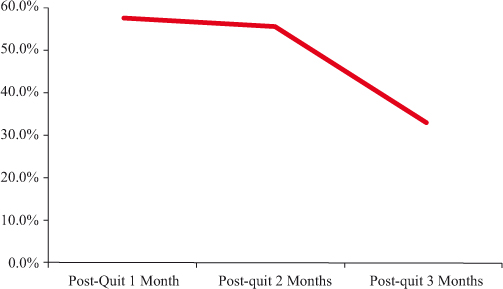
Figure 2: Seven-day point prevalence abstinence rate over time by self report
Discussion
To the best of our knowledge, this is the first smoking cessation study that conducted a home-based salivary cotinine test in real time while monitoring the whole process via a mobile-phone video call. The test was feasible and highly cost effective. Abstinence rates at post-quit 3-months showed no difference between self-reports and salivary cotinine tests, which might be affected by the small sample of the study. Of note, women in this study had been at least five times informed that they would be asked to perform the salivary cotinine test at 3-month follow-up. Thus, they might be less likely to underreport than when the test was not required. Nonetheless, five out of 20 women (25%) who self-reported abstinence could not be verified with the test and some of the women might have underreported their relapse to smoking. The rate was much higher than the non-disclosure rate (13%) found among general U.S. smokers.3
Another interesting finding from the present study was the sudden drop in abstinence rates between the second and third month of quitting. The self-reported abstinence rates did not change much (-2.0%) between the first and second month of quitting but suddenly dropped (-14.3%) between the second and third month of quitting when the salivary cotinine test was done. In contrast, most Korean American women (12/18, 66.7%) who participated in a face-to-face smoking cessation intervention study relapsed to smoking within the first month of quitting, only one (0.05%) relapsed afterward, and the remaining five maintained abstinence till the last follow-up assessment (post-quit 12 months).20 The women received the same culturally adapted smoking cessation intervention and the same weeks of nicotine patches as those in the present study. The only difference was that the study provided person-to-person cessation counseling in an office setting and self-reported abstinence was verified using a breath CO test at each follow-up assessment.
In the present study, the breath CO test was not performed at the first two follow-up assessments because the study was conducted with Korean American women recruited remotely across the nation. Individuals are likely to underreport smoking when abstinence was based on self-report only due to recall bias and social desirability.32 The possibility of underreports found in this study is troublesome given that many smoking cessation studies including quit-line services continue to rely on self-report in the evaluation of treatment effects on abstinence.33–35 The researchers based their rationale for not conducting biochemical verification on the SRNT subcommittee’s recommendations without paying attention to possible high non-disclosure rates among certain subgroups of smokers.
Findings from the present study should be interpreted with caution for the following limitations. First, the study sample was relatively small, which might have affected the non-significant difference in abstinence rates between self-report and salivary cotinine test. Second, salivary cotinine test could yield a false positive result if women in this study had been exposed to secondhand smoke, which might be the case of the woman who yielded a level of 1 (cotinine concentration 10-30ng/ml) at the retest. Third, participants in this study were Korean American women who were seeking treatment for smoking cessation and findings might not be generalizable to those who are not seeking treatment. Ample evidence exists that many Asian American women are not seeking treatment and they are as likely to underreport the use as those who are seeking.8–11 Fourth, approximately 19% of the women (18/95) who were otherwise eligible could not participate in this study due to having no access to a video call application.21
Some Korean American women erroneously believed that we were going to videotape their face during the video call. They were more likely to be those in the 50s and 60s who might be less familiar with a video call application than women under 50 years. The women were afraid that some of the videotapes could be accidentally disclosed to others who might recognize them. Thus, assuring confidentiality and security of transmitting their data is an important strategy for recruiting and retaining participants in a study utilizing a video call application. We had to explain several times that video call is different from videotaping and the present study did not entail videotaping their image.
Although the present study failed to find a significant difference in abstinence rates between self-report and biochemical test, the sudden decline at the time of the biochemical test might suggest that some women had been underreporting their smoking when abstinence was based on self-reports only. Evidence from other studies3–7 indicates that biochemical verification of smoking abstinence is necessary if we want to test accurately the efficacy of a smoking cessation intervention. Irrespective of treatment intensity, the verification should be mandated for such groups of smokers as pregnant women, youth, and minority smokers, including Asian American women, who tend to underreport use. The majority of people, especially younger people under age 50 are now using a smartphone and are familiar with a video call web application.36 Thus, conducting a home-based salivary cotinine test via a mobile-phone video call may be an ideal approach to the verification.
Acknowledgement
This work was supported by the National Institute on Drug Abuse to Sun S. Kim (1R56DA036798-01A1).
Declaration of Competing Interests
All authors had financial support from the National Institute on Drug Abuse for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
1. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. ![]()
2. Bell K, McCullough L, Salmon A, et al. Every space is claimed: smokers’ experiences of tobacco denormalization. Social Health Illn 2010;32(6):914–29. ![]()
3. Curry LE, Richardson A, Xiao H, et al. Nondisclosure of smoking status to health care providers among current and former smokers in the United States. Health Educ Behav 2013;40(3):266–73. ![]()
4. Stuber J, Galea S, Link BG. Stigma and smoking: the consequences of our good intentions. Soc Serv Rev 2009;83(4):585–609. ![]()
5. Hilberink SR, Jacobs JE, van Opstal S, et al. Validation of smoking cessation self-reported by patients with chronic obstructive pulmonary disease. Int J Gen Med 2011;4:85–90. ![]()
6. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res 2015;17(5):e113. doi:10.2196/jmir.3975. ![]()
7. Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and non-pregnant women of reproductive age in the United States. Am J Epidemiol 2011;173(3):355–9. ![]()
8. Burgess D, Fu SS, Joseph AM, et al. Understanding smoking and cessation among Hmong smokers. J Health Care Poor Underserved 2008;19(2):442–51. ![]()
9. Chen MS. The status of tobacco cessation research for Asian Americans and Pacific Islanders. Asian Am Pac Isl J Health 2001;9(1):61–5.
10. Kim SS, Kim SH, Seward G, et al. Korean American women’s experiences with smoking and factors associated with their quit intentions. ISRN Addict 2010. doi:10.1155/2013/796570. ![]()
11. Wewers ME, Dhatt RK, Moeschberger MI, et al. Misclassification of smoking status among Southeast Asian adult immigrants. Am J Respir Crit Care Med 1995;152(6 part 1):1917–21. ![]()
12. Jung-Choi KH, Khang YH, Cho HJ. Hidden female smokers in Asia: a comparison of self-reported with cotinine-verified smoking prevalence rates in representative national data from an Asian population. Tob Control 2012;21(6):536–42. ![]()
13. Jant Pharmacol Corporation. Accutest: NicAlertTM Test. Retrieved from www.accutest.net/products/pdf/DS43NicAlertSalivaInsert.pdf (accessed 25 Apr 2016)
14. Benowitz NL, Jacob P III. Metabolism of nicotine to cotinine studies by a dual stable isotope method. Clin Pharmacol Ther 1994;56(5):483–93. ![]()
15. Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. J Appl Behav Anal 2008;41(4):609–15. ![]()
16. Dallery J, Raiff BR. Contingency management in the 21st century: Technological innovations to promote smoking cessation. Substance Use Misuse 2011;46(1):10–22. ![]()
17. Meredith SE, Robinson A, Erb P, et al. A mobile-phone-based breath carbon monoxide meter to detect cigarette smoking. Nicotine Tob Res 2014;16(6):766–73. ![]()
18. Centers for Disease Control and Prevention. Disparities in adult cigarette smoking — United States, 2002–2005 and 2010–2013. MMWR 2016;65(30):753–58.
19. University of California Los Angeles. California Health Interview Survey. California Health Interview Survey, adult survey, 2015. Available at http://www.chis.ucla.edu/signup.asp
20. Kim SS, Kim SH, Fang H, et al. A culturally adapted smoking cessation intervention for Korean Americans: a mediating effect of perceived family norm toward quitting. J Immigr Minor Health 2015;17(4):1120–9. ![]()
21. Kim SS, Sitthisongkram S, Bernstein K, et al. A videoconferencing smoking cessation intervention for Korean American women: preliminary findings. Int J Womens Health 2016;8:453–62. ![]()
22. Kim SS. Predictors of short-term smoking cessation among Korean American men. Public Health Nurs 2008;25(6):516–25. ![]()
23. Kim SS, Fang H, DiFranza J, et al. Gender differences in the Fagerström Test for Nicotine Dependence in Korean Americans. J Smok Cessat 2012;7(1):31–6. ![]()
24. Suinn RM, Rickard-Figueroa K, Lew S, et al. The Suinn-Lew Asian Self-Identity Acculturation Scale: An initial report. Educ Psychol Meas 1987;47(2):401–7. ![]()
25. Leong FTL, Chou EL. Developing a brief version of the Suinn-Lew Asian Self-Identify Acculturation (SL-ASIA) Scale for counseling research. Asian Am Pac Isl J Health 1998;6(1):13–24.
26. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict 1989;86(9):1119–27. ![]()
27. Difranza JR, Savageau JA, Wellman RJ. A comparison of the autonomy over tobacco scale and the Fagerström test for nicotine dependence. Addict Behav 2012;37(7):856–61. ![]()
28. Wellman RJ, DiFranza J, Morgenstern M, et al. Psychometric properties of the Autonomy over Smoking Scale in German. Eur Addic Res 2012;18:76–82. ![]()
29. Hughes JR, Keely JP, Niaura RS, et al. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5(1):13–25. ![]()
30. Cooke F, Bullen C, Whittaker R, et al. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tobacco Res 2008;10(4):607–12. ![]()
31. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31(2):180–91. ![]()
32. McPherson S, Packer RR, Cameron JM, et al. Biochemical marker of use is a better predictor of outcomes than self-reported metrics in a contingency management smoking cessation analog study. Am J Addict 2014;23(1):15–20. ![]()
33. Barth J, Critchley J, Bengel J. Efficacy of psychosocial interventions for smoking cessation in patients with coronary heart disease: A systematic review and meta-analysis. Ann Behav Med 2006;32(1):10–20. ![]()
34. Nohlert E, Öhrvik J, Tegelberg A, et al. Long-term follow-up of a high- and a low-intensity smoking cessation intervention in a dental setting—a randomized trial. BMC Public Health 2013;13:592. ![]()
35. Zhu S-H, Cummins SE, Wong S, et al. The effects of a multilingual telephone quitline for Asian smokers: a randomized controlled trial. J Natl Cancer Inst 2013;104(4):299–310. ![]()
36. Ziefle M, Bay S. How older adults meet complexity: aging effects on the usability of different mobile phones. Behav Inf Technol 2005;24(5):375–89. ![]()

