GekoTM: a new revolution in DVT prophylaxis?
Deep vein thrombosis (DVT) is the formation of coagulated blood in one of the deep venous systems of the lower limbs, which is usually instigated by venous stasis, already-present endothelial damage of the veins and a predisposition to hypercoagulability. Despite the many advances in pharmacological prophylaxis for post-surgical DVT, it still remains a highly prevalent problem with an annual incidence of 80 cases per 100,000 and up to 50% causing life-threatening pulmonary embolism (PE)[1]. Furthermore, with the use of pharmacological prophylaxis, there is a need to balance out immediate post-surgical haemorrhage with the formation of DVT.
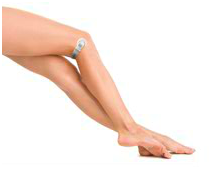
The end of days for painful subcutaneous injections is closer than we think with the creation of the GekoTM: a portable compact device resembling a wristwatch, which utilises electrical energy to stimulate the common peroneal nerve in order to activate the muscles of the lower leg that are responsible for the so-called “calf-muscle-pump” of the deep venous system. Recent pilot studies in London’s St. Bartholomew’s Hospital and Queen Mary University have demonstrated sonographic evidence of increased blood-flow at the distal femoral vein with the GekoTM device applied.[2]
GekoTM, as its name suggests, is a self-adhesive and user-friendly device that is applied to the posterior knee just at the level of the popliteal fossa. With no wires or leads it is uncombersome and patients report no discomfort or pain. No more pain from injections? Sounds like a sticky idea.
[1] Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. Mar 23 1998;158(6):585-93.
[2] (A. T. Tucker, A. Maass, D. S. Bain et al. Augmentation of venous, arterial, and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int. J. Angiol. 2010; 19 (1): e31-e37).
Official Press Release
A MEDICAL device developed by a Daresbury Science and Innovation Campus company, Sky Medical Technology, has won a major award.
The gekoTM is a wristwatch-sized device which fits to the back of the knee and stimulates blood flow in the legs – effectively mimicking the blood-flow rate normally seen when people are walking.
Bionow, the membership cluster organisation for bioscience businesses in the North West, named the gekoTM as product of the year at its annual awards at the Mere Country Club.
The highly portable device is being evaluated for a range of medical indications including the prevention of DVT in post-surgical applications where patients are immobile and at a higher risk of developing DVT.
Bernard Ross, Chief Executive, of Sky Medical Technology, said: “We are extremely excited and very proud to receive the Bionow award for product of the year. It provides important recognition of the team effort in bringing the gekoTM device to market.
“There is nothing more rewarding than being part of a focused multi-disciplined team taking a technology from concept, through design and clinical development and then onto commercialization.
“This device will ultimately provide significant benefits to patients across a range of care pathways where other forms of DVT prevention are not available and as an alternative to less cost efficient products.”
The gekoTM device is self-adhesive and easy to apply and delivers:
· One-size-fits-all increased blood circulation – which means less inventory and training
· 50 – 70% blood flow rate of walking – measured by doppler in the femoral vein in healthy volunteers
· Leading anti-stasis capability – proven to be more effective than other mechanical methods
· Mobility – no wires or leads, allowing patients to mobilize sooner
· Ease of use – operated from just one button
John Downes, chairman of Daresbury SIC and managing director at joint venture partner Langtree, said: “This kind of innovation is an exemplar of the life-changing science which goes on at Daresbury.
“Scores of businesses here are developing products and systems from research right through to commercial application and it is the combination of a diverse range of science and technology businesses, plus the world-leading facilities afforded by the Science and Technology Facilities Council, which enables such developments to take place.”
The recently approved SCI-TECH enterprise zone will reinvest business rates to deliver new specialist office, laboratory and technical space, with the potential to create as many as 10,000 skilled jobs and leverage more than £150m in private sector investment.
Science and technology businesses that start up or relocate to the SCI-TECH enterprise zone will qualify for a 100 per cent business rate discount for five years, up to a maximum of £275,000 over five years.
CE Marked under the Medical Devices Directive, the gekoTM device is not approved by the US FDA and not available for sale in the USA.

