Clinical performance of the HeartBuds, an electronic smartphone listening device, compared to FDA approved Class I and Class II stethoscopes
Ritesh S. Patel1, Julio Schwarz, MD1, Valerie Danesh, RN2, Arnold Einhorn, MD3, David Bello, MD1,3
1University of Florida College of Medicine, USA; 2Orlando Health Corporate Office of Research Operations, Clinical Research Coordinator in Critical Care, USA; 3Orlando Health Heart Institute, USA
Corresponding Author: rspatel1@ufl.edu
Journal MTM 5:1:45–51, 2016
Background: Auscultation with stethoscopes is essential to the physical exam. However, the stethoscope has not appreciably changed since Leared and Cammann developed the first binaural stethoscopes in the mid 1800s. Technological advances make it possible to use smartphone technology to auscultate patients. The HeartBuds, a listening device that integrates with an iPhone app, achieves this purpose.
The purpose of this study was to compare HeartBuds’ acoustic superiority over the FDA approved class I blue disposable stethoscopes, which are commonly used in practice to reduce hospital infection rates, and demonstrate equivalence to the gold standard FDA class I analog stethoscope, the Littmann Cardiology III, and the FDA class II digital stethoscope, the Littmann Electronic 3200.
Methods: 50 adult patients were auscultated with each of the above-mentioned stethoscopes by two independent examiners. They rated their acoustic quality and completed surveys documenting body sounds heard.
Results: The disposable stethoscope was significantly worse at identifying cardiac murmurs (p < 0.002), and performed poorly when auscultating for carotid bruits (p < 0.058). The HeartBuds stethoscope was equivalent to its more commonly used counterparts, the Littmann Cardiology III and the Littmann Electronic 3200. Examiners also found it to be of comparable acoustic quality to these models.
Conclusion: HeartBuds is a smartphone compatible listening device that was superior in examining cardiovascular sounds to approved FDA Class I disposable stethoscopes, and equivalent to FDA approved class I and class II Littmann stethoscopes. Considering HeartBuds equivalence to more expensive stethoscopes while costing much less, the HeartBuds can potentially reduce infection rates without sacrificing quality.
Background
Auscultation is essential to physical examination. All physicians have learned how to listen to patients using a stethoscope, and most physicians use auscultation in everyday practice. Auscultating patients has origins in ancient Egyptian medicine. Arthur Leared and George Cammann developed the binaural stethoscopes familiar today in the mid 1800s. Now, medicine is at a crossroads between reducing costs and improving quality. Technology available today can help achieve this, and the stethoscope is a medical device that is ready for such innovation.
Furthermore, a Mayo Clinic study1 examined physician stethoscope bacterial concentrations and found they facilitate the horizontal transmission of millions of the bacteria that place acutely ill patients at risk for hospital acquired drug resistant infections, including MRSA. It supports numerous others before it, which have observed that stethoscopes are frequently contaminated with enterococci,2 Staphylococcus aureus, and other Gram-positive bacteria.3 One major vector of infection is the stethoscope, yet physicians use them with thousands of patients yearly, often with poor compliance in sanitizing them between each patient interaction.4
Millions of stethoscopes are sold every year in the USA ranging from low quality, low cost disposable stethoscopes to higher quality, more expensive stethoscopes used by cardiologists and other healthcare professionals that demand a high acoustic quality level. The FDA classifies analog stethoscopes as Class I medical devices, and newer model electronic stethoscopes are classified as Class II medical devices. Mobile phone devices used for healthcare applications are also considered Class II medical devices.
This quality study introduces the HeartBuds electronic stethoscope, a personal auscultatory device that uses the Apple iPhone® and a physician’s own headphones to record and auscultate a patient. By using the advanced sound processors designed to listen to distant sounds (Audience, Inc., Apple, Inc.) already built into “Siri enabled” iPhones (iPhone 4S and newer), the HeartBuds system bypasses requiring expensive internal electronics and as a result, costs about $6 to produce per unit. In contrast, the Littmann Electronic 3200 has a retail price of approximately $397 and the Cardiology III has a retail price of approximately $155.
In a recent in vivo study comparing HeartBuds to the Littmann Electronic 3200 and two passive disposable stethoscopes, HeartBuds demonstrated higher overall sensitivity than the other models, and both electronic stethoscopes demonstrated superiority in allowing auscultation of sounds of very high and low frequencies, making it suitable for auscultating cardiovascular and valvular sounds.5
This study focuses on demonstrating HeartBuds’ acoustic equivalence to the traditional stethoscopes used by many physicians and medical students today, and superiority to the blue disposable stethoscopes commonly used in hospitals today to reduce risk for nosocomial infection.
Materials and Methods
This clinical quality study compared the Littmann Cardiology III, a class I FDA approved medical device, Littmann Electronic 3200, a class II FDA approved medical device, and a generic disposable stethoscope commonly used in hospital wards, which is also a class I FDA approved medical device to the HeartBuds system (Figure 1). Two examiners were used with each patient, each performing a cardiac, pulmonary, and abdominal auscultative exam with each patient participant. Good auscultatory technique was performed per Bates Guide to Physical Examination.6
Figure 1: Stethoscopes used in daily practice
For the cardiac exam, patients were auscultated for carotid bruits, and murmurs in the aortic, pulmonic, tricuspid, and mitral positions. For the pulmonary exam, patients were auscultated on their chest and back for abnormal breathing sounds. For the abdominal exam, examiners listened for bowel sounds and the presence of abdominal vascular bruits.
Participants were selected to participate blindly, during scheduled office visits in a cardiology and a pulmonology clinic based in Orlando, FL. Neither participants nor examiners were aware of any auscultatory pathophysiology. Patients with documented auscultatory pathophysiology, patients unwilling to participate, and patients younger than 18 years of age were excluded from this study. Each examiner individually auscultated and recorded his findings from each device, documenting what sounds were heard and making a quality measurement using a Likert scale, 1–5, 1 being the worst quality and 5 being the highest quality. The quality measures were averaged for each examiner for each type of auscultatory exam, cardiovascular, pulmonary, abdominal, and carotid bruits.
Both examiners auscultated with the disposable stethoscope first, because of the expectation that this device would be the least sensitive, and then they were free to listen with the other three devices in any order they please. 50 patients were auscultated to allow for sufficient numbers of abnormal findings, which would aid in sensitivity analysis for these devices.
For the Littmann Electronic 3200 stethoscope, examiners were allowed to adjust the volume settings to levels they were comfortable with. Likewise, examiners were allowed the same with the HeartBuds unit. Examiners plugged the HeartBuds device into their personal iPhone 5S, loaded with the HeartBuds app available on the App Store. Examiners used Apple EarPods, which plugged directly into the HeartBuds unit. These are the standard headphones supplied with Apple iPhones today (Figure 2).

Figure 2: Apple EarPods and iPhone 5S
Data was collected using surveys, which contained questions about the sounds heard specific to the cardiac, pulmonary, abdominal, and carotid examinations. Each examiner was given four surveys to fill out, one per device. Data was gathered into two separate Excel documents, which were then organized and combined for data collection.
Kappa coefficients and paired student T-test were calculated to compare inter-rater reliability and device comparability, respectively. Device quality was calculated based on means of the examiner Likert scores for each device.
The study was approved by the Institutional Review Board (IRB) at Orlando Health.
Results
The results show that device quality perception varied little between examiners for each of the devices, and kappa coefficients demonstrate high interrater reliability between devices: 0.85 for cardiac murmurs (Table 1, Table 2). The HeartBuds (range: 3.2–3.45) was of comparable quality to the Littmann Electronic 3200 (range: 3.52–4.17) and Cardiac III model stethoscopes (range: 3.41–3.48).

Table 1: Mean Device Quality (50 patients)
Table 2: Interrater Reliability (Kappa values)
Compared to the disposable stethoscopes, the two Littmann models and the HeartBuds stethoscope were much more effective in auscultating cardiac murmurs (Table 3). Likewise, the three advanced models were more effective in auscultating carotid bruits than the disposable stethoscope (Table 4). All four stethoscopes had comparable performance when auscultating adventitious pulmonary sounds and abdominal sounds (Table 5, Table 6).
Table 3: Cardiovascular Murmurs Auscultated (n=50)
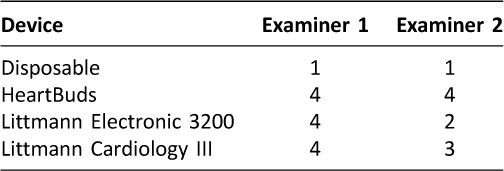
Table 4: Carotid Bruits Auscultated (n=50)
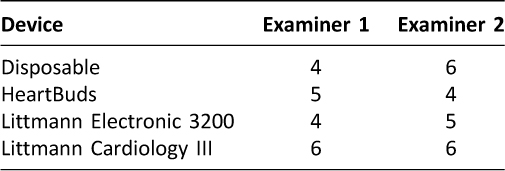
Table 5: Pulmonary Adventitious Sounds Auscultated (n=50)
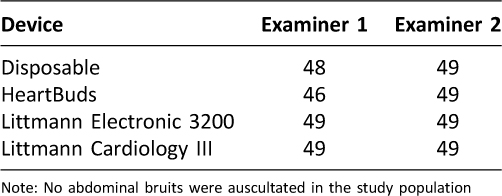
Table 6: Abdominal Sounds Auscultated (n=50)
A paired T-test was performed using the Littmann Cardiology III as the gold standard measurement (Table 7). For auscultating cardiac murmurs, the two Littmann models and the HeartBuds stethoscope were comparably effective in detecting murmurs, while the disposable stethoscope was significantly worse (p < 0.002). Auscultations of pulmonary and abdominal sounds were not significantly different between the four stethoscopes.
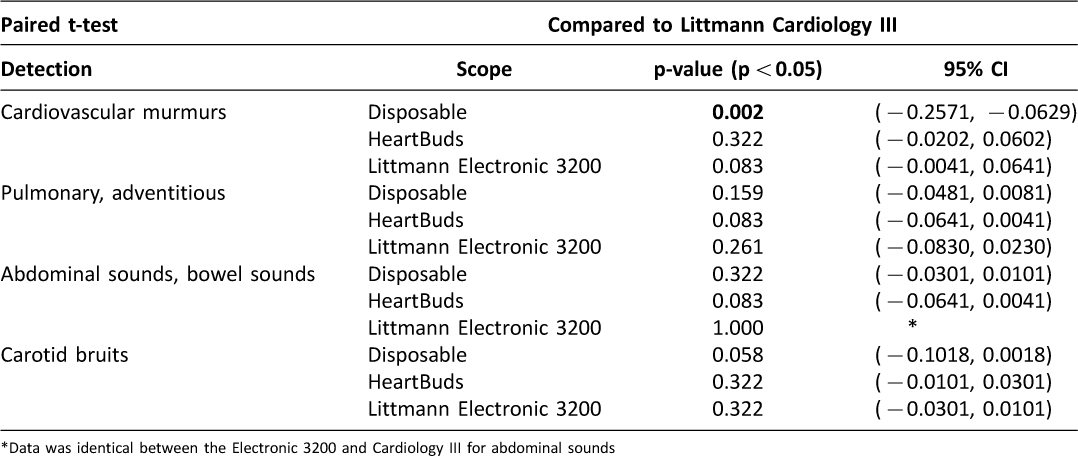
Table 7: Differences between stethoscope models in detecting physiologic sounds, compared against Littmann Cardiology III
Discussion
The results indicate that disposable stethoscopes (FDA class I) are of significantly lower quality when auscultating heart sounds (p < 0.002) and for carotid bruits (p < 0.058) than the other three devices tested. HeartBuds is equivalent to the gold standard stethoscopes used by many physicians today, the Littmann Cardiology III (FDA class I) or the Littmann Electronic 3200 (FDA class II).
When considering the raw data in a study population of 50 patients, approximately 43% of cardiac murmurs were missed if examiners were using the disposable stethoscope compared to the Littmann Cardiology III, Littman Electronic 3200 or HeartBuds. It is interesting to note that there were two patients whose bruits were picked up by the HeartBuds stethoscope, but not by the Littmann Electronic 3200. This could be due to examiner error, or it could be related to the HeartBuds larger range of acoustic recording, which exceeds the acoustic range of the Littmann Electronic 32005. There was no difference in performance between devices for abdominal and pulmonary sounds, presumably because these sounds are coarse and easily distinguished compared to pathophysiological heart sounds.
Similarly, the disposable stethoscope did not allow examiners to pick up carotid bruits in 66% (Examiner 2)-75% (Examiner 1) of patients. With a larger sample size, the researchers are confident that the disposable stethoscopes would perform statistically worse than the other models when examining for carotid bruits. Examiners also judged the quality of the HeartBuds to be superior to disposable stethoscopes. However, the Littmann stethoscope models were rated higher in quality, though the difference was small compared to the perceived quality difference between the disposable stethoscope and these three models. The researchers believe this difference in quality could be due to the prototype device not being wireless. It often got tangled, and the device itself has no onboard electronics or batteries that would add a little more weight to the device and give it more heft in an examiner’s hand. Future device models will contain Bluetooth antennas and a rechargeable battery. Both additions would address this issue.
A laboratory audio analysis proved the electronic stethoscopes (HeartBuds and Littmann Electronic 3200) to be acoustically superior to the two low cost disposable stethoscopes tested5. Electronic stethoscopes have about 10 times higher sound amplification than the passive stethoscopes tested. Passive stethoscopes of all types have tubing that resonates at a low frequency, which allows them to pick up low frequency sounds best at the expense of sounds at all other frequencies. Electronic stethoscopes have little to no tubing contributing to sound amplification, and as such, can pick up higher frequency sounds more easily.
The researchers theorize that the sound processing technology aboard Apple’s iOS products is helpful in making HeartBuds good at picking up heart sounds. More specifically this technology was originally developed to pick up distant vocal sounds, and as such it would aid in auscultating distant body sounds as well. By using technology already available in many people’s pockets, this product can be implemented at a cost effective level yet maintain a high level of quality.
Conclusion
This study has numerous clinical implications due to vigorous interest in mobile health and its role in healthcare innovation. The HeartBuds can be a low cost, high quality substitute for higher cost devices. By being remotely used from any iPad or iPhone device, cardiac sounds can be recorded and replayed in a similar manner to current heart rhythm monitors found in cardiac wards today. The researchers believe that the HeartBuds can be a part of a wireless auscultation solution that will eliminate stethoscope surfaces available to transfer bacteria between patients.
Because it is comparable in cost to the disposable stethoscopes examined, it is conceivable that the HeartBuds can be used in the clinical setting to improve auscultative quality while reducing nosocomial infection risks. The researchers theorize that compliance with procedures involving disposable stethoscope use is low due to its poor quality, thereby undermining its purpose as a tool to reduce infection risk. Future studies with this device will attempt to prove the HeartBuds can reduce hospital infection rates due to horizontal transmission of bacteria via stethoscopes.
Overall, HeartBuds has the potential to reduce infection risk while being of comparable quality to stethoscopes that cost hundreds of dollars. Healthcare professionals may not be getting what they pay for by trusting the stethoscopes they know and love, and the rapidly evolving field of mobile health is proving this starting with the HeartBuds.
References
1. Longtin Y, et al. “Contamination of Stethoscopes and Physicians’ Hands After a Physical Examination”. Mayo Clinic Proceedings March 2014;89(3):291–9. ![]()
2. Gopinath KG, Stanley S, Mathai E, Chandy GM. “Pagers and stethoscopes as vehicles of potential nosocomial pathogens in a tertiary care hospital in a developing country”. Trop Doct. 2011;41(1):43–5. ![]()
3. Marinella MA, Pierson C, Chenoweth C. “The stethoscope. A potential source of nosocomial infection?”. Arch Intern Med. 1997;157(7):786–90. ![]()
4. Bukharie HA, Al-zahrani H, Rubaish AM, Abdulmohsen MF. “Bacterial contamination of stethoscopes”, J Family Community Med. 2004;11(1):31–3.
5. Drzewiecki, G, Katta, H, Pfahnl, A, Bello, D, Dicken, D. “Active and Passive Stethoscope Frequency Transfer Functions”, IEEE Signal Processing in Medicine and Biology Symposium. Philadelphia, 12/2014.
6. Bickley, L. S., Szilagyi, P. G., & Bates, B. (2007). Bates’ guide to physical examination and history taking: Lynn S. Bickley, Peter G. Szilagyi. Philadelphia: Lippincott Williams & Wilkins.

