Posted on Sep 22, 2012 in News |
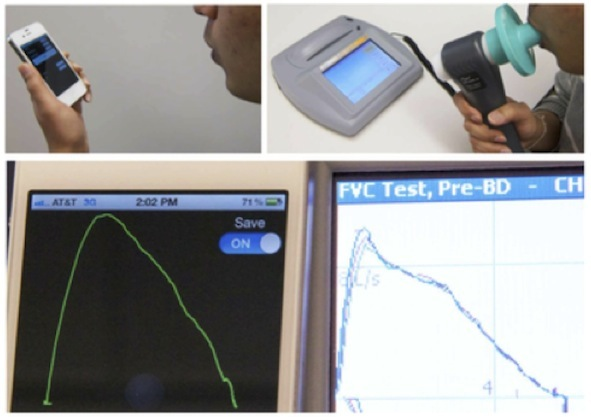
A spirometer is a device used mainly by respiratory physicians to diagnose and objectively monitor the lung function of patients suffering from common conditions such as chronic obstructive pulmonary disease, asthma and cystic fibrosis. Traditionally, this device works via a differential pressure transducer to determine the flow and thus the amount of air that is exhaled as the patient breathes into the machine. The recent announcement of SpirosmartTM by the University of Washington Department of Medicine and Seattle Children’s Hospital has been a pivotal step towards the management of chronic lung conditions.
SpirosmartTM is a mobile phone based system that extrapolates lung function using the in-built microphone. As the user inhales and exhales within a hands-breadth away from the mobile device, the software estimates the lung function measurements via a set of complicated algorithms involving the resonance frequencies of the user’s vocal tract. Initial pilot studies by the development team involving 52 patients have shown a mean error of 5.1% compared to a clinical spirometer. The current cost of the software is still under wraps, however due to its portability and ease of use, this device may be a breath of fresh air to home monitoring of chronic lung conditions and detecting exacerbations before they become worse.
Link:
http://ubicomplab.cs.washington.edu/wiki/SpiroSmart
Posted on Sep 16, 2012 in News |
 The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 3, published in September 2012. Mobile technology in Medicine is a rapidly developing area, and we hope to continue accelerating research in the field. We look forward to your submissions for Issue 4.
The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 3, published in September 2012. Mobile technology in Medicine is a rapidly developing area, and we hope to continue accelerating research in the field. We look forward to your submissions for Issue 4.
Volume 1, Issue 3 Contents
Editorials
001 Principles of m-Health survey design
R. Chakrabarti
Original Articles
006 Validation of Near Eye Tool for Refractive Assessment (NETRA) – Pilot Study
A. Bastawrous, C. Leak, F. Howard, V. Kumar
017 A Meta-Analysis of Mobile Health and Risk Reduction in Patients with Diabetes Mellitus: Challenge and Opportunity
L. Liu, S. Ogwu
025 Ambulatory Autonomic Activity Monitoring Among At-Risk Adolescent Mothers
S. Rajan, N. Leonard, R. Fletcher, B. Casarjian, R. Casarjian, C. Cisse, M. Gwadz
032 A Preliminary Investigation of the Benefits and Barriers to Implementing Health Information Technology in Medical Clinics
A. Chesser, N. Woods, J. Wipperman
040 Using Mobile Tethering for sharing data across devices: application in rural eye screening
G. Kong, J. Kam
Case Reports
046 Application of self-recorded photos using mobile phones in maxillofacial surgery
F. Pourdanesh, A. Sayyedi, A. Jamilian, M. Yaghmaei
Letters
050 Ethical Considerations Related to Mobile Technology Use in Medical Research
M. Parker
053 The use of short message service (SMS) for patient appointment reminders
S. McClean, M. Perera
In keeping with our open-access principles, all articles are published both as full text and as PDF files for download. For your convenience, attached to this post is a PDF file containing the complete Volume 1, Issue 3, which can be easily downloaded and saved for viewing offline.
We look forward to hearing from readers in the comments section, and encourage authors to submit research to be considered for publication in this peer-reviewed medical journal.
Yours Sincerely,
Editorial Board
Journal of Mobile Technology in Medicine
Posted on Sep 16, 2012 in Editorial |
..
Dr Rahul Chakrabarti MBBS1,2
1 Editor-In-Chief, Journal of Mobile Technology in Medicine, 2Centre for Eye Research Australia
Corresponding Author: rahul@journalmtm.com
Journal MTM 1:3:1-5, 2012
doi:10.7309/jmtm.16
Before you start
The first step is to conduct a critical appraisal of existing literature relevant to the research question. Before proceeding further, it is then necessary to consider whether a survey is the most appropriate method to collect the data required to answer the research question. Appropriate alternatives to surveys include a systematic review or meta-analysis, case-studies, or studies with focus groups. It is beyond the scope of this article to discuss these alternate forms in depth, however, the researcher should be guided by published literature in their topic. The key steps for survey design will now be discussed. (Refer to figure 1).
Posted on Sep 16, 2012 in Original Article |
Dr Andrew Bastawrous1,2, Dr Christopher Leak2, Frederick Howard3, Mr B Vineeth Kumar1
1Wirral University Teaching Hospitals NHS Foundation Trust, UK, 2International Centre for Eye Health, Clinical Research Department, Faculty of Infectious & Tropical Diseases, London School of Hygiene and Tropical Medicine, UK, 3Independant Optometrist, UK
Corresponding Author: Andrew.bastawrous@lshtm.ac.uk
Journal MTM 1:3:6-16, 2012
DOI:10.7309/jmtm.17
Background: Uncorrected-refractive-error (URE) is the leading cause of global visionimpairment (VI); 122.5 million people are estimated VI from URE. NETRA is a $30USD clip-on application for smartphones.
Purpose: To validate the NETRA as an alternative to subjective refraction for potential use in resource-poor countries.
Methods: NETRA uses a pinhole mask attached to a smartphone displaying a spatially resolved pattern to the subject. Refractive error is estimated by the patient subjectively aligning patterns by a touchscreen interface on the smartphone. NETRA was compared to subjective refraction in 34 eyes.
Results: The mean Subjective Spherical Equivalent (SSE) was -0.65D (std 2.79, 95%CI ±0.97) Two-sided T-test showed that mean SSE is not statistically significantly different (two sided t-test; t=1.6742 p=0.1036, 95% CI±0.29) from the mean NETRA Spherical Equivalent (NSE) . Mean difference of Spherical Equivalents (NSE – SSE) was 0.24D (Std 0.84, 95%CI ±0.29). And NETRA produced a mean VA improvement of 0.44LogMAR (Std 0.52, 95%CI±0.18), or four Snellen lines.
Conclusion:In settings where access to a trained refractionist is not possible, NETRA has the potential to estimate refractive error closely enough to render an individual no longer VI from URE. NETRA is potentially a cost-effective tool in meeting the VISION2020 goals to eradicate avoidable blindness and warrants further testing in resource-poor settings.
Posted on Sep 16, 2012 in Original Article |
Dr Longjian Liu PHD1, Stella-Maris Ogwu MPH1,2
1Department of Epidemiology and Biostatistics, Drexel University School of Public Health, Philadelphia, USA, 2Opening Doors for Health Disparity Training Porgram, Drexel University School of Public Health, Philadelphia, USA Corresponding Author: longjian.liu@drexel.edu
Journal MTM 1:3:17-24, 2012
DOI:10.7309/jmtm.18
Purpose: To examine scientific evidence on the effectiveness of mobile phone technology in Diabetes Mellitus (DM) care management.
Methods: A systematic review was conducted through literature searches from three electronic databases and was restricted to English-language articles published between January 2002 and March 2012. Studies that used mobile phone intervention and reported changes in diet, physical activity, and blood glucose and /or glycosylated hemoglobin (HbA1c) levels were retrieved. A meta-analysis was conducted for studies with HbA1c measures.
Results: More than 50 articles were screened. Of them, 15 met the review criteria. Of the 15, study sample sizes ranged from 12 to 130 participants aged 8 to 70 years old. Duration of intervention ranged from 1 to 12 months. Overall, significant improvements were observed in blood glucose and/or HbA1c concentration, adherence to medication, healthy lifestyle, and self-efficacy. Twelve of 15 trials, which had serum HbA1c measures, showed an average 0.39% (95%CI: -0.067, -0.721) HbA1c reduction from studies with pre- to post-tests (p=0.018).
Conclusion: Findings from the study provide the evidence that health reminders, disease monitoring and management, and education through mobile phone technology may significantly help improve glycaemic control patients with DM.
Posted on Sep 16, 2012 in Original Article |
Dr Sonali Rajan EdD1, Dr Noelle Leonard PhD1,2, Dr Richard Fletcher PhD3 Dr Beth Casarjian PhD4, Robin Casarjian MA4, Cathleen Cisse MPH24, Dr MaryaGwadz PhD2
1Teachers College, Columbia University, New York, USA,2New York University, College of Nursing, New York, USA,3Massachusetts Institute of Technology, Cambridge, USA, 4Lionheart Foundation, Boston, USA
Corresponding Author: sr2345@tc.columbia.edu
Journal MTM 1:3:25-31, 2012
DOI:10.7309/jmtm.19
Background: Many adolescent mothers experience significant challenges in regulating emotions due to adverse life experiences, which can place adolescent mothers and their children at risk for poor developmental outcomes. Ambulatory monitoring of stress that also provides immediate feedback using wearable biosensors has the potential to enhance clinician-delivered parenting interventions and help young mothers develop emotion regulatory skills.
Methods: We conducted a pilot study to assess the acceptability, ease of use, and preliminary efficacy of a wearable biosensor, the iCalm sensor band, among a sample of four mothers, ages 15-18 years. Mothers wore the biosensor for a period of 24-36 hours while engaging in normal, daily tasks (e.g. caring for their child, attending school). Both quantitative electrodermal activity (EDA) data (via the iCalm sensor band) and qualitative data (via individual semi-structured interviews) were collected.
Results: The adolescent mothers were able to comfortably use and wear the iCalm sensor band. EDA data were collected and corresponded with stressful daily life events described by the mothers during qualitative interviews.
Conclusion: The iCalm biosensor is acceptable to use among high-risk adolescent mothers and appears to help mothers with the development of emotion regulatory skills.







 The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 3, published in September 2012. Mobile technology in Medicine is a rapidly developing area, and we hope to continue accelerating research in the field. We look forward to your submissions for Issue 4.
The Editorial Board at the Journal of Mobile Technology in Medicine is proud to present Volume 1, Issue 3, published in September 2012. Mobile technology in Medicine is a rapidly developing area, and we hope to continue accelerating research in the field. We look forward to your submissions for Issue 4.

